Abstract
Background
Clinical experience has shown that a single measure is not sufficient to assess disease activity in rheumatoid arthritis (RA). Various clinimetric tools are necessary to address the many clinical situations that can arise.
Methods
In order to develop a comprehensive measurement tool, the Pan American League of Associations for Rheumatology searched for the most frequent measures of disease activity applied in RA by means of a semi-systematic review of the available literature.
Results
We found that the most frequently reported measures of disease activity were the 28-joint Disease Activity Score, C-reactive protein, and the erythrocyte sedimentation rate, followed by patient-reported measures of pain and stiffness and many other composite indices and patient-reported outcome measures. The most frequent physician-reported sign of disease was the swollen joint count, and the most frequently self-reported feature was the increase in disease activity or flares.
Conclusion
In this article, we present a new clinimetric tool developed based on expert consensus and on data retrieved from our search. Disease activity can be better assessed by combining various data sources, such as clinical, laboratory, and self-reported outcomes. These variables were included in our novel clinimetric tool.
Key Points • The goal of treatment of RA is to achieve the best possible control of inflammation, or even remission; therefore, disease management should include systematic and regular evaluation of inflammation and health status. • Clinimetric tools evaluate a series of variables (e.g., symptoms, functional capacity, disease severity, quality of life, disease progression) and can reveal substantial prognostic and therapeutic differences between patients. • Our clinimetric tool, which is based on a combination of data (e.g., clinical variables, laboratory results, PROMs), can play a relevant role in patient assessment and care. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that affects between 0.5 and 1% of the general population, with a higher prevalence in industrialized countries [1, 2]. The prevalence of RA has increased over the past three decades, although the severity of the disease has been declining steadily, probably because of changes in treatment paradigms and better management. However, RA still carries a significant burden for patients, public health, and society [1,2,3,4,5].
Clinimetrics was first introduced and defined in the 1980s as a “discipline aimed at creating indices, rating scales and other expressions to describe or measure symptoms, physical signs and other clinical phenomena” [6]. Clinimetrics also includes the psychosocial impact of disease and treatment on the individual, their family, and interpersonal relationships and on daily activities and well-being [6, 7].
The expansion of the assessment process to include the psychological, emotional, and social impact of RA has led to more widespread use of biopsychosocial perspectives and patient-reported data in our approach to the disease [8,9,10,11]. Clinimetric tools evaluate symptoms, functional capacity, disease severity, timing of clinical changes, impact of comorbidities, quality of life, and progression of illness and can reveal substantial prognostic and therapeutic differences between patients [9,10,11,12].
Disease activity measures such as the 28-joint Disease Activity Score (DAS28), the Simplified Disease Activity Index (SDAI), and the Clinical Disease Activity Index (CDAI) cover the most relevant aspects of RA in a single score [12,13,14,15]. DAS28 is used in clinical trials and routine monitoring to evaluate response to treatment and inform decisions on the need to begin or adjust treatment [12,13,14,15,16,17,18,19].
Several outcome measures have proven to be helpful in daily practice, clinical trials, and clinical epidemiology [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Many variables used to assess the activity of RA are based on disease activity measures and patient-reported outcome measures (PROMs) [17,18,19,20,21,22,23,24,25,26,27,28].
In RA, the challenge to precisely evaluate the activity of disease includes having to perform a complete physical examination, which can be difficult, particularly in situations where telemedicine is required. Previous studies have found this method of patient care to be non-inferior to regular consults [29]. But since most of the available clinimetric tools require a physician’s examination, PROMs become more relevant in order to promptly adjust treatment aiming towards the remission of the activity of disease [18,19,20, 30].
We describe the development of a novel comprehensive clinimetric tool to record relevant symptoms of RA and their impact on patients. The tool has the potential to be used in both daily practice and clinical trials. Therefore, at the Pan American League of Associations for Rheumatology (PANLAR), we performed a semi-systematic review for current information on clinimetrics and their use in RA by using a search strategy as well as by including articles deemed relevant to clinical practice. We then confirmed the validity and reliability of our instrument in clinical practice. This part of our study is ongoing and will be reported on in the future.
Methods
Phase I: literature review
We conducted a semi-systematic literature review of clinical measurements used to establish disease activity in RA patients. The literature review was conducted in Medline using the following search strategy: ((“Patient Outcome Assessment”[Mesh]) AND (“Patient Reported Outcome Measures” [Mesh])) AND (“Arthritis, Rheumatoid”[Majr]). The search was restricted to papers published in the last 5 years.
Titles of retrieved articles were screened to define whether they were suitable for our purposes, and those that were considered inadequate were excluded. This initial search was followed by similar screening processes to evaluate the abstracts in a second stage and, finally, the full text. At each of these stages, texts that did not meet the inclusion criteria were excluded. Additional articles that were relevant to the objectives of our paper were subsequently added based on the judgment and experience of the authors.
Phase II: identification and categorization of disease activity measures
We extracted the disease activity measures reported in each article and determined the number of articles that mention each one. Next, we divided the disease activity measures into categories based on whether they required medical input, whether they might be used when a physical examination is not possible, and whether they were laboratory test values and clinical findings suggesting disease activity.
The findings were categorized into the following groups:
-
Physician’s assessment of the disease
-
Patient’s self-assessment of the disease (i.e., PROMs)
-
Tender joint count (TJC)
-
Swollen joint count (SJC)
-
Laboratory tests
-
Morning stiffness
-
Pain
-
Self-report of flare
-
Full assessment scales
The full set of items found in literature as well as their frequency of appearance can be found in Table 1.
Phase III: consensus-based development of a clinimetric tool
After collecting and categorizing the variables of interest, we invited experts in rheumatology to participate in a consensus using the nominal group technique. The goal of this consensus was to determine the variables that were the most indicative of disease activity for each category according to the experts’ opinion. For patient-reported outcomes, the variables chosen were those that did not require the physician’s input.
Seventeen experts accepted the invitation to participate. Two rounds were carried out. The first round was to collect individual responses, and the second round was to share a summary of the responses provided by other participants. Finally, a vote was held in two synchronous sessions using a digital platform.
The basic questionnaire consisted of 12 sections that began with the question: “Which item or items would you include in a clinimetric tool for use in telemedicine or data collection in the waiting room?” followed by answer options based on the categories described in phase II. The voting process was always anonymous, and the summary of answers was subsequently sent to other experts as feedback. Once the voting process was complete, the responses were grouped by frequency according to the information provided by the experts for each group of questions.
Results
Literature review
The initial literature search retrieved 234 titles. Studies that did not fulfill the search objectives were excluded. After a review of titles, abstracts, and full texts, the final set included 92 articles. Figure 1 shows a flow diagram of the records included and excluded (Fig. 1).
Screening process. Based on: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. https://doi.org/10.1136/bmj.n71
The reasons for excluding articles at each of the three screening stages (title, abstract, and full-text review) are shown in the Appendix.
Disease activity measures
The most frequently reported measures of disease activity included in the final set were the DAS28, which was reported in 26.18% of articles, C-reactive protein (CRP) in 21.89%, and the erythrocyte sedimentation rate (ESR) in 13.30% (Table 1).
Within the activity indices assessed by the physician, we found 14 activity measures that required the physician’s evaluation: the most frequently reported were DAS28 (31.77%) and the CDAI (12.5%).
We identified 12 PROMs. The most common were the Patient Global Assessment (PtGA) (6.77%) and the Routine Assessment of Patient Index Data (RAPID3) (3.65%). Within this category, the most widely reported laboratory test was CRP (61.45%).
Across all the categories, the most frequently reported sign of disease was the SJC (52.38%). The most frequently self-reported symptom was increased activity or flare (26.53%).
Consensus-based development of a clinimetric tool
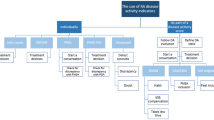
With the results of the consensus and the vote, we created a comprehensive clinimetric instrument that brought together the variables that ranked highest in the voting process. The resulting clinimetric tool was named PAtient Reported Disease Activity Index in Rheumatoid Arthritis (PARDAI-RA) and can be seen in Fig. 2.
Discussion
We collected up-to-date information on clinimetric instruments and their usefulness in RA through a semi-systematic search. This information was then used to design a comprehensive clinimetric instrument based on the most frequently used measures of disease activity (both physician- and patient-reported).
The development of a clinimetric tool for rheumatic diseases is a complex process that requires various steps to be followed [7,8,9,10,11, 31,32,33]. Several tools have been developed in recent decades (e.g., the Rheumatoid Arthritis Distress Scale of Silke et al. [32] and the Rheumatoid Arthritis Symptom and Impact Questionnaire of Becker et al. [33]), although there is still an unmet need for new measures.
The clinimetric perspective facilitates clinical decision-making. Implementation of these decisions is likely to improve outcomes both in clinical research and in practice. As new treatments and clinical concepts are constantly being developed, novel instruments and tests are needed to provide meaningful information that complements the work of the clinician. In any given medical context, including rheumatology, measurements must be accurate before tests are applied. Clinimetric properties indicating that the test is reliable and valid are essential when determining the measurement quality of any tool [6,7,8,9,10,11].
The clinimetric tools used in RA differ from those used in other clinical conditions because there is no single “gold standard” measure that can be applied to all patients. The use of multiple health domains in RA has led to the development of composite indices consisting of different quantitative measures that improve clinical evaluations by reducing measurement error, thus providing a more objective means of assessment [6,7,8,9, 9, 10, 10, 11, 11,12,13,14,15,16,17,18,19].
There is consensus that inflammation in RA should be controlled as soon and as completely as possible in clinical practice. Considering that the goal of treatment is to achieve the best possible control of inflammation, or even remission, the management of RA should include systematic and regular evaluation of inflammation and health status. Control of the long-term consequences, especially disability and joint damage, is a key objective in the clinical management of RA [16,17,18,19,20]. Our comprehensive clinimetric tool could help clinicians reach these therapeutic goals.
For the purposes of our study, we evaluated the most widely recommended composite measures of disease activity. The most common metric was the DAS28, which indicates how active RA is at the moment of the evaluation, and how it will progress over time. DAS28 has been extensively validated and is widely considered the best option for measuring disease activity in RA. It is endorsed by the American College of Rheumatology and the European League Against Rheumatism for clinical trials [13, 14]. The instrument was initially designed for comparing clinical trial outcomes of RA treatments, although these indices are now also used as overall markers of disease activity in daily clinical practice [15,16,17,18,19].
Response to treatment can be assessed more objectively using the TJC, SJC, or DAS evaluations. The DAS28-ESR describes the severity of RA using clinical and laboratory data and may be combined with a general health evaluation or a Patient Global Assessment (PtGA) [12,13,14,15,16,17,18,19].
We also found the CDAI and SDAI to be widely used. The CDAI combines single measures into an overall continuous measure of disease activity. It includes the 28 SJC, the 28 TJC, a PtGA based on a 10-cm visual analog scale (VAS), and the Physician Global Assessment, which is also based on a 10-cm VAS. SDAI has been extensively validated and has shown high sensitivity and specificity for predicting how physicians will modify therapy. Both indices are well-known and widely used in research and in clinical settings [10, 12,13,14,15, 19].
C-reactive protein (CRP) proved to be an appropriate alternative to ESR for assessing disease activity. Therefore, we also included CRP in our clinimetric tool. Many experts consider CRP to be a more direct measure of inflammation than ESR, with faster increases when inflammatory damage appears. Hence, CRP is considered valid as ESR for measuring the activity of RA. ESR and CRP are both associated with radiological progression in RA and are extremely useful in the monitoring of disease activity. Since the activity of RA can fluctuate between visits, close monitoring is helpful, especially when exacerbation is related to radiologic progression and structural changes.
In the development of our clinimetric tool, we gave considerable relevance to patient-reported variables. PROMs represent a significant advance in the assessment of RA. They are used as secondary outcomes in most clinical trials and are recognized as measures of treatment efficacy by the US Food and Drug Administration and the European Medicines Agency [13, 14]. Combining PROMs with objective measures provides essential insight into treatment effects and global health status [17,18,19,20].
PROMs allow patients to report their symptoms directly, although their role in assessing inflammation and joint damage may be imprecise. Symptom severity (e.g., pain and stiffness), global patient assessment, physical function, and global quality of life are essential outcomes in RA that can be measured using PROMs [20,21,22,23,24,25,26,27,28].
PROMs also enable the impact of treatment to be consistently quantified from the patient’s perspective and complement physician-reported measures, such as joint counts and laboratory data. Therefore, our tool included two scales to assess pain and morning stiffness from the patient’s perspective.
Conclusion
Various indices, scales, and scores are needed for the assessment of patients living with RA, since clinical experience has shown that a single metric is unlikely to capture changes in disease activity across all RA patients in various clinical situations. Therefore, the development of a comprehensive clinimetric tool is an appropriate and essential step towards better care and management of RA patients.
In research and daily practice, disease activity is assessed using a combination of data, such as clinical variables, laboratory testing, and patient-reported variables. These categories of variables were included in our novel clinimetric tool, thus supporting its relevance in patient assessment and care.
References
Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, Alpizar-Rodriguez D, Lauper K (2022) Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol 18(10):591–602. https://doi.org/10.1038/s41584-022-00827-y
Scott IC, Whittle R, Bailey J, Twohig H, Hider SL, Mallen CD, Muller S, Jordan KP (2022) Rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis epidemiology in England from 2004 to 2020: an observational study using primary care electronic health record data. Lancet Reg Health Eur 23:100519. https://doi.org/10.1016/j.lanepe.2022.100519
van Delft ETAM, Jamal M, den Braanker H, Kuijper TM, Hazes JMW, Lopes Barreto D, Weel-Koenders AEAM (2022) A systematic review on time trend incidence of rheumatoid arthritis in outpatient rheumatology clinics. Front Med (Lausanne) 9:933884. https://doi.org/10.3389/fmed.2022.933884
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41(5):863–877. https://doi.org/10.1007/s00296-020-04731-0
Fernández-Ávila DG, Rincón-Riaño DN, Bernal-Macías S, Gutiérrez Dávila JM, Rosselli D (2019) Prevalencia de la artritis reumatoide en Colombia según información del Sistema Integral de Información de la Protección Social. Rev Colomb Reumatol 26:83–87
Fava GA (2022) Forty Years of Clinimetrics. Psychother Psychosom 91:1–7. https://doi.org/10.1159/000520251
Berrocal C, Cosci F (2022) XII National Congress of the Research Group in Psychosomatics (RGP) October 21 and 22, 2022 - the clinimetric method. Clin Ter 173(Suppl 1(5)):1–92. https://doi.org/10.7417/CT.2022.2451
Te Molder MEM, Vriezekolk JE, Bénard MR, Heesterbeek PJC (2021) Translation, cross-cultural adaptation, reliability and construct validity of the Dutch Oxford Knee Score - activity and participation questionnaire. BMC Musculoskelet Disord 22(1):700. https://doi.org/10.1186/s12891-021-04521-0
Corominas H, Millan AM, Diaz-Torne C (2020) Rheumatoid arthritis: defining clinical and ultrasound deep remission. Mediterr J Rheumatol 31(4):384–388. https://doi.org/10.31138/mjr.31.4.384
Kardas T, Wielosz E, Majdan M (2022) Methods of assessment of joint involvement in various systemic connective tissue diseases. Reumatologia 60(1):53–62. https://doi.org/10.5114/reum.2022.114186
Nielsen LM, Oestergaard LG, Kirkegaard H, Maribo T (2021) Construct validity and clinical utility of World Health Organization disability assessment schedule 2.0 in older patients discharged from emergency departments. Front Rehabil Sci 2:710137. https://doi.org/10.3389/fresc.2021.710137
Salaffi F, Di Carlo M, Farah S, Marotto D, Atzeni F, Sarzi-Puttini P (2021) Rheumatoid arthritis disease activity assessment in routine care: performance of the most widely used composite disease activity indices and patient-reported outcome measures. Acta Biomed 92(4):e2021238. https://doi.org/10.23750/abm.v92i4.10831
England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, O’Dell JR, Ranganath VK, Limanni A, Suter LG, Michaud K (2019) 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken) 71(12):1540–1555. https://doi.org/10.1002/acr.24042
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Díaz E, Cajas J, Casallas A, Abella J, Morales R, Rondón F et al (2020) Measurement of overall perceived health using different scales in patients with rheumatoid arthritis: a proposal of a combined scale. Rev Colomb Reumatol 27:262–268
Molina Collada J, Trives L, Castrejón I (2021) The importance of outcome measures in the management of inflammatory rheumatic diseases. Open Access Rheumatol 13:191–200. https://doi.org/10.2147/OARRR.S276980
Fleischmann R, Haraoui B, Buch MH, Gold D, Sawyerr G, Shi H, Diehl A, Lee K (2022) Analysis of disease activity metrics in a methotrexate withdrawal study among patients with rheumatoid arthritis treated with tofacitinib plus methotrexate. Rheumatol Ther. https://doi.org/10.1007/s40744-022-00511-3
Brkic A, Łosińska K, Pripp AH, Korkosz M, Haugeberg G (2022) Remission or not remission, that’s the question: shedding light on remission and the impact of objective and subjective measures reflecting disease activity in rheumatoid arthritis. Rheumatol Ther 9(6):1531–1547. https://doi.org/10.1007/s40744-022-00490-5
Aletaha D, Wang X, Zhong S, Florentinus S, Monastiriakos K, Smolen JS (2020) Differences in disease activity measures in patients with rheumatoid arthritis who achieved DAS, SDAI, or CDAI remission but not Boolean remission. Semin Arthritis Rheum 50(2):276–284. https://doi.org/10.1016/j.semarthrit.2020.03.022
Bugatti S, De Stefano L, Manzo A, Sakellariou G, Xoxi B, Montecucco C (2021) Limiting factors to Boolean remission differ between autoantibody-positive and -negative patients in early rheumatoid arthritis. Ther Adv Musculoskelet Dis 13:1759720X211011826. https://doi.org/10.1177/1759720X211011826
Küçükdeveci AA, Elhan AH, Erdoğan BD, Kutlay Ş, Gökmen D, Ateş C, Yüksel S, Lundgren-Nilsson A, Escorpizo R, Stucki G, Tennant A, Conaghan PG (2021) Use and detailed metric properties of patient-reported outcome measures for rheumatoid arthritis: a systematic review covering two decades. RMD Open 7(2):e001707. https://doi.org/10.1136/rmdopen-2021-001707
Horta-Baas G (2022) Patient-reported outcomes in rheumatoid arthritis: a key consideration for evaluating biosimilar uptake? Patient Relat Outcome Meas 13:79–95. https://doi.org/10.2147/PROM.S256715
Pickles T, Macefield R, Aiyegbusi OL, Beecher C, Horton M, Christensen KB, Phillips R, Gillespie D, Choy E (2022) Patient reported outcome measures for rheumatoid arthritis disease activity: a systematic review following COSMIN guidelines. RMD Open 8(1):e002093. https://doi.org/10.1136/rmdopen-2021-002093
Bartlett SJ, De Leon E, Orbai AM, Haque UJ, Manno RL, Ruffing V, Butanis A, Duncan T, Jones MR, Leong A, Perin J, Smith KC, Bingham CO (2020) Patient-reported outcomes in RA care improve patient communication, decision-making, satisfaction and confidence: qualitative results. Rheumatology (Oxford) 59(7):1662–1670. https://doi.org/10.1093/rheumatology/kez506
Bingham CO, Butanis AL, Orbai AM, Jones M, Ruffing V, Lyddiatt A, Schrandt MS, Bykerk VP, Cook KF, Bartlett SJ (2021) Patients and clinicians define symptom levels and meaningful change for PROMIS pain interference and fatigue in RA using bookmarking. Rheumatology (Oxford) 60(9):4306–4314. https://doi.org/10.1093/rheumatology/keab014
Renskers L, Rongen-van Dartel SA, Huis AM, van Riel PL (2020) Patients’ experiences regarding self-monitoring of the disease course: an observational pilot study in patients with inflammatory rheumatic diseases at a rheumatology outpatient clinic in The Netherlands. BMJ Open 10(8):e033321. https://doi.org/10.1136/bmjopen-2019-033321
Provan SA, Michelsen B, Sexton J, Uhlig T, Hammer HB (2020) Trajectories of fatigue in actively treated patients with established rheumatoid arthritis starting biologic DMARD therapy. RMD Open 6(3):e001372. https://doi.org/10.1136/rmdopen-2020-001372
Bartlett SJ, Gutierrez AK, Andersen KM, Bykerk VP, Curtis JR, Haque UJ, Orbai AM, Jones MR, Bingham CO 3rd (2022) Identifying minimal and meaningful change in a patient-reported outcomes measurement information system for rheumatoid arthritis: use of multiple methods and perspectives. Arthritis Care Res (Hoboken) 74(4):588–597. https://doi.org/10.1002/acr.24501
Avouac J, Molto A, Frantz C, Wanono S, Descamps E, Fogel O, Combier A, Poiroux L, Miceli-Richard C, Allanore Y (2022) Evaluation of patients with rheumatoid arthritis in teleconsultation during the first wave of the COVID-19 pandemic. J Rheumatol 49(11):1269–1275. https://doi.org/10.3899/jrheum.220073
Duarte C, Ferreira RJO, Santos EJF, da Silva JAP (2022) Treating-to-target in rheumatology: theory and practice. Best Pract Res Clin Rheumatol 36(1):101735. https://doi.org/10.1016/j.berh.2021.101735
Thiele K, Albrecht K, Zink A, Aringer M, Karberg K, Späthling-Mestekemper S, von Hinüber U, Callhoff J (2022) Is the Rheumatoid Arthritis Impact of Disease (RAID) score a meaningful instrument for other inflammatory rheumatic diseases? A cross-sectional analysis of data from the German National Database. RMD Open 8(2):e002342. https://doi.org/10.1136/rmdopen-2022-002342
Silke L, Kirresh O, Sturt J, Lempp H (2021) Development of the Rheumatoid Arthritis Distress Scale (RADS): a new tool to identify disease-specific distress in patients with Rheumatoid Arthritis. BMC Rheumatol 5(1):51. https://doi.org/10.1186/s41927-021-00220-4
Becker B, Bracher M, Chauhan D, Rendas-Baum R, Lin X, Raymond K, O’Connor M, Kosinski M (2021) Development, psychometric evaluation and cognitive debriefing of the rheumatoid arthritis symptom and impact questionnaire (RASIQ). J Patient Rep Outcomes 5(1):129. https://doi.org/10.1186/s41687-021-00400-3
Acknowledgements
PANLAR Investigation Unit. Editorial assistance was provided by Content Ed Net.
Funding
Open Access funding provided by Colombia Consortium This work did not have external funding and was developed with PANLAR’s own financial resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Ávila, D.G., Patiño-Hernández, D., Moreno-Luna, S. et al. Development of a novel clinimetric tool: PAtient Reported Disease Activity Index in Rheumatoid Arthritis (PARDAI-RA) by PANLAR, for the assessment of patients living with rheumatoid arthritis. Clin Rheumatol 43, 1277–1285 (2024). https://doi.org/10.1007/s10067-024-06868-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-06868-w