Abstract
Introduction/objectives
systemic sclerosis (SSc) is an autoimmune disorder with multiple organs destruction. This study aimed to identify the ultrasonographic changes of major salivary glands in Egyptian scleroderma patients and to detect their association to different disease manifestations.
Methods
Forty-seven SSc patients and 43 apparent healthy volunteers were enrolled. Demographics, inflammatory markers, and autoimmune status were recorded. Ultrasound evaluation of salivary glands was performed. Salivary gland changes’ associations were statistically examined with SSc susceptibility and disease manifestations.
Results
Thirty-one SSc patients exhibited glandular pathology (p < 0.0001), compared to controls. Of these abnormalities, SSc patients showed a total parotid gray scale of 2, total submandibular gray scale of 2, total glandular gray scale of 4, and total glandular Doppler signal of 1 at p < 0.0001, compared to the control group. Patients with SSc and glandular pathology had a higher prevalence of arthritis (p = 0.029) and ESR (p = 0.002) than those with normal glandular ultrasound. Significant associations were reported between gray scale ultrasound (GSUS) of total parotid (odds ratio “OR” = 0.4), total submandibular (OR = 0.36), and total glandular (OR = 0.53) with susceptibility to SSc at p < 0.0001. Total glandular GSUS (p = 0.039) and total submandibular power Doppler (p = 0.044) correlated with the SSc duration. Total parotid GSUS (p = 0.008) and total glandular GSUS (p < 0.0001) correlated with Schirmer’s test.
Conclusions
Major salivary glands are affected in SSc. Hence, scanning these glands with ultrasound is an additive tool besides the current practice.
Key Points • Major salivary gland changes, observed by ultrasonography, are new findings in Egyptian SSc patients. • Ultrasound changes of major salivary glands are associated with inflammatory markers and clinical manifestations of SSc. • Scleroderma ultrasonography scans of the main salivary glands could be added to the routine work. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a multi-organ autoimmune disease in which the pathological inflammatory process, vasculopathy, and fibrosis cause multiple disease manifestations [1]. Typically, it progresses destructively to several organs, including the skin, joints, gastrointestinal system, heart, lungs, and kidneys. In the first 1 or 2 years of the disease, 40% of patients experience organ damage that may be irreversible and affects one or more organs [2]. Sicca symptoms are common in SSc patients. In previous years, few studies reported the involvement of abnormalities in salivary and lacrimal glands in patients with SSc that resulted in hyposalivation and dry eye complaints [3, 4]. Since the publication of Alarcón-Segovia and his colleagues in 1973 who described SSc as the most elusive among all connective tissue diseases. They concluded that minor salivary glands changes are due to collagen deposition, which is related to the pathological process in SSc, and they found that ductal changes are related to autoimmunity. Hence, Sjogren syndrome (SS) was implicated making this disease the most baffling among connective tissue diseases [5]. Several studies followed with conflict results being a natural course of SSc pathogenesis or is relation to associated SS [6,7,8,9]. The mechanism of sicca manifestations in SSc is believed to be due to the fibrosis process of the salivary glands (SGs) [10, 11]. SGs represent the target organ for autoimmunity in SS [12]. SS manifests in the exocrine glands resulting in sicca symptoms and other systemic extra-glandular manifestations including cutaneous, musculoskeletal, renal, pulmonary, hematological, and neurological affection [13]. The disease course may be discrete, but it can coexist with autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus [14, 15]. Secondary Sjogren in systemic sclerosis has been investigated seldom with limited data available, with reported frequencies of 7.5%, 14%, 23%, up to 28% [9, 10, 16,17,18].
Nowadays, ultrasound (US) scan in rheumatic diseases is an additional clinical skill for many health practitioners that requires long-term training and practice [19]. Ultrasonography of the salivary gland is an emerging tool in recent decades with excellent opportunities and has many advantages [9, 20]. A subtask force of the Outcome Measures in Rheumatology Clinical Trials (OMERACT) working group studied the use of salivary gland ultrasound as a possible outcome instrument for the measurement of salivary gland abnormalities in patients with primary Sjogren syndrome (pSS) [21]. There is no international consensus for defining these abnormalities in patients with SSc.
The aforementioned studies prompted us to investigate whether or not our Egyptian scleroderma patients’ main salivary glands changed, and whether or not these changes correlated with clinical symptoms and signs, inflammatory markers, and autoantibodies.
Patients and methods
Patients
This case-control study was conducted at the outpatient clinics of rheumatology from April 2022 to October 2022. Forty-seven Egyptian adult patients with systemic sclerosis were enrolled in the current study. SSc patients were diagnosed according to the American College of Rheumatology (ACR)/European Alliance of Rheumatology Associations (EULAR) [22]. They were classified into limited and diffused systemic sclerosis according to LeRoy’s criteria [23]. Forty-three apparent healthy volunteers were involved and served as a control group. Juvenile cases, pregnant, lactating mothers, individuals with a history or current; hepatitis C and human immunodeficiency viral infection, lymphoma, graft versus host disease, amyloidosis, diabetics, other rheumatic diseases, and those with history of parotitis were excluded. Also, subjects exposed to radiation and individuals who received anticholinergic drugs were excluded. The study was conducted according to the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board, Faculty of Medicine, Minia University (Serial No. 312-2022). Signed consent was obtained from all participants.
Clinical and laboratory evaluation
Data collected from all the participants include age, gender, disease duration at the time of presentation, current treatments received, clinical characteristics, cutaneous manifestations, musculoskeletal manifestations, joint symptoms, Raynaud’s phenomenon, digital ulcers or scars, and interstitial lung disease (ILD). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were measured in all subjects as markers for inflammation. All participants were screened for autoimmune profiling through a check for antinuclear antibody (ANA), rheumatoid factor (RF), anti-Ro60/SSA, anti-La/SSB, anti-topoisomerase 1 (anti-topo I), and anti-centromere (ACA). The presence of sicca symptoms was checked by a standard questionnaire [24]. Schirmer’s test without anesthesia [25] was applied at the same setting of glandular scanning. Finally, all participants were exposed to an assessment of skin tightness using the modified Rodnan total skin score [26].
Ultrasound examination of salivary gland
Evaluation of the salivary glands was performed by experienced rheumatologists who are EULAR, certified in musculoskeletal US with more than 7 years’ experience at the same sitting for clinical evaluation and laboratory sampling.
Examination was performed according to EULAR standardized procedures for ultrasound imaging in rheumatology [27]. AM and HA used a 14 MHz Toshiba xario-200 from Japan; RA and AH used an 8 MHz to 12 MHz South Korean Albinion Cube 8 Diamond; MM and RE used a 10 MHz to 18 MHz American AH LOGIC E 10 GE. All machines were equipped with power Doppler (PD) detection and common specifications including frequency range of 5–10 MHz, a low–medium wall filter, and a pulse repetition frequency of 400–1000 Hz were commonly adjusted for the involved machines.
The major salivary glands (both parotids and both submandibular) were scanned. Scanning was done by gray scale ultrasound (GSUS) and PD in two planes; longitudinal and transverse. During scanning, patient head was turned opposite to the scanned side to scan the parotids then hyperextended to visualize the submandibular glands while the patient was advised to relax supinated [6]. Regarding GSUS scanning, a semiquantitative 4 grades US was applied according to the OMERACT recommendations [21], each salivary gland was scored on (0–3) scoring system by GSUS [15]. That score depends on glandular inhomogeneity and the presence of hypo/anechoic areas. Normal salivary glands are homogenous echotexture granular pattern in ultrasound comparable to thyroid echogenicity and its visualized as hyperechoic structure when compared to nearby tissues, with frequent normal vasculatures. G0, normal; G1, mild inhomogeneity with no anechoic/hypoechoic areas; G2, moderate inhomogeneity with focal anechoic/hypoechoic areas; G3, diffuse inhomogeneity with anechoic/hypoechoic areas occupying the entire gland surface or fibrous echo-structures. The presence of hyperechoic bands on the gland surface, which evolves into fibrotic tissue, characterizes the fibrous formations. A 0–12 total OMERACT salivary gland ultrasound (SGUS) grade was calculated as the sum of the single grades of the four glands. Each pair of glands was also given a score between zero and six. Intraglandular PD was also graded from zero to 3 according to the parenchymal blood flow. Glands were scored 0 if there is no flow, graded as 1 if there is up to three isolated signals, if the parenchymal flow was seen in < 50% of the echotexture it is G2 and if its > 50% then G3 will be applied [20]. According to previous records [28], a salivary gland GSUS score ≥ 2 was considered pathological. Hence, sub-classification was applied into pathological (if ≥ 2) and nonspecific if (< 2) in one salivary gland (parotid or submandibular).
Statistical analysis
The power of the study was computed utilizing G*Power 3.1.9.7 software (Franz Faul, Universität Düsseldorf, Germany) (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The test family was adjusted at F test and the statistical test used was adjusted at ANCOVA: fixed effects, main effect, and interactions. The input parameters were effect size F = 0.4, β/α ratio = 2, total sample size = 90, numerator df = 10, number of groups = 2, and number of covariates = 1. All statistical analyses were performed using the Statistical Package for Social Science version 26 for Windows (SPSS software package, Chicago, USA). The tests of data normality were statistically determined using the Kolmogorov–Smirnov test with Lilliefors significance correction. The categorical data were expressed as frequencies (percentages) and nonparametric data were expressed as median (interquartile range). The nonparametric data were examined between the SSc group and control group using Mann–Whitney statistical analysis. χ2 test was utilized to assess the differences in the categorial data between SSc patients and the control individuals.
Binary logistic regression analysis was operated to conduct the power of the association of salivary gland abnormalities with the susceptibility to SSc disease compared to the control subjects. The power of associations was quantified by the odds ratio (OR) with a 95% confidence interval (CI) adjusted for age and gender. Multiple linear regression analysis was used to investigate the association of the independent variables relative to the dependent variables after transforming nonparametric variables into the logistic scale. The regression models were established using the “enter” analysis. All p values were 2-sided, and a p value < 0.05 was considered statistically significant. For association analyses, the statistical significance thresholds were set to p < 0.0125 after Bonferroni correction.
Results
The demographics of all participants are listed in Table 1. The control subjects were age and sex matched chosen to be matched in age with the SSc group (P value = 0.665). The frequency of male and female gender was 9.3 and 90.7%, respectively, in the control group. On the other hand, the frequency was 12.80% for males and 87.20% for females among SSc patients. Patients had SSc disease for a median of 48 months. A percentage of 17.00 of SSc patients had a limited cutaneous SSc disease, while 83.00% had a diffused cutaneous SSc. Among SSc patients, 61.70% showed xerostomia, 59.60% had xerophthalmia, 85.11% showed sicca symptoms in the form of dry eye and/or dry mouth, 70.20% had arthritis, 74.50% had interstitial lung disease (ILD), and 48.90% had fingers ulcer/scars, compared to the control group (p value < 0.0001). Furthermore, all SSc patients had Raynaud’s phenomenon (100%). Of SSc patients, 38.30% had a pathological Schirmer’s test (< 5 mm, p < 0.0001), compared to the control group. SSc patients showed a high skin score with a median of 22. In addition, SSc patients received different treatments either alone or in combination; 24 patients received colchicine therapy, 25, 21, 13, and 4 patients treated with steroid, methotrexate, cyclophosphamide, and mycophenolate mofetil, respectively (p value < 0.0001).
Clinical and laboratory features of SSc patients, compared to the control subjects, are represented in Table 2. There were significant elevations in the inflammatory markers (ESR and CRP) among SSc patients, compared to the control individuals (p < 0.0001). Moreover, 74.50% of SSc patients were positive for ANA test with high titer (p < 0.0001), compared to the control group. Only 34 and 10.60% of SSc patients showed positive results for anti-topo I (p < 0.0001) and ACA (p = 0.028) antibodies, respectively, compared to the control group. Regarding anti-Ro and anti-La antibodies, 17.00 and 14.90% among SSc patients showed positive anti-Ro (p = 0.005) and anti-La (p = 0.008) tests, respectively, compared to the control group. Of SSc patients, 78.70% showed negative results for both anti-Ro and anti-La tests, while 21.30% were positive for any of them (p < 0.0001).
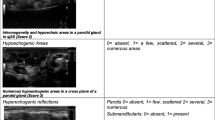
The changes in the homogeneity of the salivary gland, observed by the ultrasound in the studied groups, are represented in Fig. 1a–f. These changes were observed by 6 observers with strong inter-rater Kappa agreement of 0.79–0.86. Among the SSc group, 31 patients exhibited glandular pathology (p < 0.0001), compared to the control individuals. Of these abnormalities, SSc patients showed a total parotid gray scale of 2, total submandibular gray scale of 2, total glandular gray scale of 4, and total glandular Doppler signal of 1 at p < 0.0001, compared to the control group (Fig. 1g).
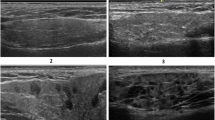
Representative ultrasound images showing four-grade semiquantitative scoring system of salivary gland. a GSUS of the parotid (grade 2). b PDUS of the parotid gland (grade 1). c GSUS changes of the submandibular gland (grade 1). d PDUS of the submandibular gland (grade 2). e GSUS of the submandibular gland (grade 3). f PDUS of the submandibular gland (grade 3). g Data are expressed as median (interquartile range) for nonparametric variables and frequencies (percentages) for categorical variables. SSc, systemic sclerosis; GS, gray scale; DS, Doppler signal; GSUS, gray scale ultrasound; PDUS, power Doppler ultrasound. A single asterisk (*) denotes significance at P < 0.0001, compared to the control individuals. The mean difference is significant at p < 0.05 using asymptotic significance 2-sided
Data shown in Table 3 illustrates the difference between SSc patients with and without glandular pathology regarding the clinical and laboratory features. There were no statistical differences in all clinical and laboratory features between SSc patients with and without the glandular pathology except for the presence of arthritis and ESR values. Of SSc patients, 80.60% with glandular pathology had arthritis (p = 0.029) in association with having a higher ESR value (50%, p = 0.002) than those with normal glandular US.
Results in Table 4 showed significant associations of salivary gland abnormalities (total parotid GS “OR = 0.40, 95% CI = 0.25–0.64,” total submandibular GS “OR = 0.36, 95% CI = 0.22–0.60,” and total glandular GS “OR = 0.53, 95% CI = 0.39–0.73”) with the risk of SSc, compared with the control group, at p value < 0.0125 after the Bonferroni correction. However, the total glandular Doppler signal did not show a significant association with the risk of SSc, compared to the control group (p value = 0.994).
Table 5 describes the importance of the observed salivary gland abnormalities as independent variables for SSc duration, Schirmer’s test, skin score, and ANA titer as dependent variables among all individuals. The salivary gland abnormities showed significant correlations only with SSc duration and Schirmer’s test. Both total glandular GS (β = 0.37, t = 2.16 at p = 0.039) and total submandibular PD (β = − 0.38, t = − 2.12 at p = 0.044) showed significant correlations with the SSc duration. On the other hand, both total parotid GS (β = − 0.43, t = − 2.83 at p = 0.008) and total glandular GS (β = − 0.50, t = − 4.15 at p < 0.0001) showed highly significant correlations with Schirmer’s test.
Discussion
Scleroderma is a systemic autoimmune disease characterized by extensive fibrosis, vasculopathy together with abundant autoantibodies. Vasculopathy is the initial process in scleroderma that affects almost all organs leading to ischemic changes. Progressive fibrosis gradually disrupts skin and internal organ architecture and leads to organ dysfunction [29]. The hyposecretory function of salivary glands is well documented in several autoimmune diseases; Sjogren’s syndrome, rheumatoid arthritis, autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, primary biliary cirrhosis, and chronic graft versus host disease with different explanatory theories [30]. The current study was set to record ultrasound abnormalities in the major salivary glands of Egyptian patients with SSc and to detect if there is an association between these changes and different disease manifestations.
The current study illustrated a high prevalence of Egyptian SSc patients suffering from sicca symptoms. In addition, they had positive Schirmer’s test in a significant number of them. All of which agree with the previous reports [6, 11, 31]. Furthermore, SSc patients were characterized by the elevations in the inflammatory markers (ESR and CRP) which is in line with Lakota et al. [32]. In agreement with previous studies [6], the autoimmunity status expressed by ANA, anti-topo I, ACA, anti-Ro, and anti-La positivity was recorded among SSc patients.
In addition to the typical symptoms of SSc, such as interstitial lung disease, Raynaud’s phenomenon, arthritis, fingers ulcer/scars, and sicca symptoms, two-thirds of our patients also displayed novel ultrasound findings, including salivary glandular alterations in echogenicity and enhanced PD signals in the glandular echotexture which are also recently observed in the SSc patients [6, 9]. Regarding the clinical and laboratory manifestations, SSc patients with glandular pathology differ from those with normal glandular features only in having arthritis with higher ESR values. This might explain that the incidence of severe inflammation among SSc patients can be related to the incidence of salivary gland abnormalities observed among these patients. Previous studies showed that ESR is increased in SSc and predicts mortality [33, 34]. ESR is one of the parameters incorporating the modified Medsger SSc disease severity scale [35, 36]. The occurrence of a systemic inflammatory process in SSc patients had been reported [32] due to elevating levels of circulating inflammatory cytokines and chemokines which are associated with internal organ involvement and allow the discrimination between limited and diffuse forms of SSc [37]. These cytokines and chemokines are also reported to be related to the incidence of salivary gland abnormalities [38].
As far as we know, no reports statistically analyzed the association between salivary gland abnormalities and susceptibility to SSc disease. The current study reports a strong association of total parotid GS, total submandibular GS, and total glandular GSUS with the existence of the disease. To the best of our knowledge, there are no previous studies that have reported the relationship between salivary gland US abnormalities and different disease parameters in SSc patients. A close association was reported in the current study between both total glandular GSUS and total submandibular PD on one side and the SSc duration on the other side. Also, associations of both total parotid GSUS and total glandular GSUS were recorded with the Schirmer’s test.
Conclusion
In conclusion, major salivary glands ultrasound changes observed by the gray scale and the power Doppler in Egyptian patients with SSc are exciting new findings and linked to disease susceptibility, various clinical signs and symptoms, and inflammatory markers. These results emphasize the value of ultrasound as a quick, non-invasive bedside investigation to recognize significant abnormalities of the salivary glands in SSc patients. Future studies could be performed on a larger sample of SSc patients with limited and diffuse subtypes. Different cytokines and chemokines may be evaluated in SSc patients with and without salivary gland abnormalities. Moreover, other studies on other autoimmune diseases could be conducted to differentiate these changes in Egyptian patients.
Data availability
Data are available under reasonable request to the corresponding author.
References
Denton CP (2015) Systemic sclerosis: from pathogenesis to targeted therapy. Clin Exp Rheumatol 33:S3-7
Hao Y, Hudson M, Baron M, Carreira P, Stevens W, Rabusa C et al (2017) Early mortality in a multinational systemic sclerosis inception cohort. Arthritis Rheum 69(5):1067–1077. https://doi.org/10.1002/art.40027
Benz K, Baulig C, Knippschild S, Strietzel FP, Hunzelmann N, Jackowski J (2021) Prevalence of oral and maxillofacial disorders in patients with systemic scleroderma—a systematic review. Int J Environ Res Public Health 18(10):5238. https://doi.org/10.3390/ijerph18105238
Kreps EO, Carton C, Cutolo M, Cutolo CA, Vanhaecke A, Leroy BP et al (2019) Ocular involvement in systemic sclerosis: a systematic literature review, it’s not all scleroderma that meets the eye. Semin Arthritis Rheum 49(1):119–125. https://doi.org/10.1016/j.semarthrit.2018.12.007
Alarcón-Segovia D, Ibánez G, Hernández-Ortíz J, Velázquez-Forero F, González-Jiménez Y (1974) Sjögren’s syndrome in progressive systemic sclerosis (scleroderma). Am J Med 57(1):78–85. https://doi.org/10.1016/0002-9343(74)90771-2
Lee K-A, Choi W, Kim J, Kim H-S (2021) High prevalence of salivary gland ultrasound abnormalities in systemic sclerosis. Joint Bone Spine 88(2):105113. https://doi.org/10.1016/j.jbspin.2020.105113
Zimmermann F, Robin F, Caillault L, Cazalets C, Llamas-Gutierrez F, Garlantezec R et al (2023) Sicca syndrome in systemic sclerosis: a narrative review on a neglected issue. Rheumatology (Oxford) 62(SI):SI1–SI11. https://doi.org/10.1093/rheumatology/keac412
Coiffier G, Jousse-Joulin S, Cornec D, Garlantézec R, Bleuzen A, Diot E et al (2020) Ultrasonographic salivary gland evaluation in systemic sclerosis: is sicca syndrome secondary to an authentic overlap syndrome or another specific fibrotic manifestation of the disease? Ann Rheum Dis 79(12):e160–e160. https://doi.org/10.1136/annrheumdis-2019-215972
Couderc M, Tournadre A, Mathieu S, Pereira B, Soubrier M, Dubost JJ (2020) Do the salivary glands of patients with systemic sclerosis show ultrasonographic modifications suggestive of Sjögren’s syndrome? Ann Rheum Dis 79(10):e137–e137. https://doi.org/10.1136/annrheumdis-2019-215777
Avouac J, Sordet C, Depinay C, Ardizonne M, Vacher-Lavenu M, Sibilia J et al (2006) Systemic sclerosis–associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheumatol 54(7):2243–2249. https://doi.org/10.1002/art.21922
Kobak S, Oksel F, Aksu K, Kabasakal Y (2013) The frequency of sicca symptoms and Sjögren’s syndrome in patients with systemic sclerosis. Int J Rheum Dis 16(1):88–92. https://doi.org/10.1111/j.1756-185X.2012.01810.x
Negrini S, Emmi G, Greco M, Borro M, Sardanelli F, Murdaca G et al (2022) Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med 22(1):9–25. https://doi.org/10.1007/s10238-021-00728-6
Mavragani CP, Moutsopoulos HM (2014) Sjögren syndrome. CMAJ 186(15):E579–E586. https://doi.org/10.1503/cmaj.122037
Abd-Allah NM, Hassan AA, Omar G, Hamdy M, Abdelaziz STA, Abd El Hamid WM et al (2020) Dry eye in rheumatoid arthritis: relation to disease activity. Immunol Med 43(2):92–97. https://doi.org/10.1080/25785826.2020.1729597
La Paglia GMC, Sanchez-Pernaute O, Alunno A, Martínez-Becerra MJ, Romero-Bueno F, Recuero S et al (2020) Ultrasound salivary gland involvement in Sjogren’s syndrome vs. other connective tissue diseases: is it autoantibody and gland dependent? Clin Rheumatol 39:1207–1215. https://doi.org/10.1007/s10067-019-04780-2
Tseng C-C, Yen J-H, Tsai W-C, Ou T-T, Wu C-C, Sung W-Y et al (2015) Increased incidence of Sjogren’s syndrome in systemic sclerosis: a nationwide population study. Autoimmunity 48(7):438–444. https://doi.org/10.3109/08916934.2015.1045583
Baldini C, Mosca M, Della Rossa A, Pepe P, Notarstefano C, Ferro F et al (2013) Overlap of ACA-positive systemic sclerosis and Sjögren’s syndrome: a distinct clinical entity with mild organ involvement but at high risk of lymphoma. Clin Exp Rheumatol 31(2):272–280
Salliot C, Mouthon L, Ardizzone M, Sibilia J, Guillevin L, Gottenberg J-E et al (2007) Sjögren’s syndrome is associated with and not secondary to systemic sclerosis. Rheumatology (Oxford) 46(2):321–326. https://doi.org/10.1093/rheumatology/kel252
Villaseñor-Ovies P, Navarro-Zarza JE, Canoso JJ (2020) The rheumatology physical examination: making clinical anatomy relevant. Clin Rheumatol 39:651–657. https://doi.org/10.1007/s10067-019-04725-9
Jousse-Joulin S, Nowak E, Cornec D, Brown J, Carr A, Carotti M et al (2017) Salivary gland ultrasound abnormalities in primary Sjögren’s syndrome: consensual US-SG core items definition and reliability. RMD Open 3(1):e000364. https://doi.org/10.1136/rmdopen-2016-000364
Jousse-Joulin S, d’Agostino MA, Nicolas C, Naredo E, Ohrndorf S, Backhaus M et al (2019) Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: an OMERACT ultrasound working group reliability exercise. Ann Rheum Dis 78(7):967–973. https://doi.org/10.1136/annrheumdis-2019-215024
Van Den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheumatol 65(11):2737–2747. https://doi.org/10.1002/art.38098
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger T Jr et al (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15(2):202–205
Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM et al (1993) Preliminary criteria for the classification of Sjögren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 36(3):340–347. https://doi.org/10.1002/art.1780360309
Wróbel-Dudzińska D, Kubik-Komar A, Rykwa D, Kosior-Jarecka E, Żarnowski T, Chałas R (2021) The use of Schirmer strips to measure salivary and lacrimal flow in non-Sjögren patients. Clin Oral Investig 25:4107–4114. https://doi.org/10.1007/s00784-020-03741-3
Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L et al (2017) Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2(1):11–18. https://doi.org/10.5301/jsrd.5000231
Möller I, Janta I, Backhaus M, Ohrndorf S, Bong DA, Martinoli C et al (2017) The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann Rheum Dis 76(12):1974–1979. https://doi.org/10.1136/annrheumdis-2017-211585
Damjanov N, Milic V, Nieto-González JC, Janta I, Martínez-Estupiñan L, Serrano B et al (2016) Multiobserver reliability of ultrasound assessment of salivary glands in patients with established primary Sjögren syndrome. J Rheumatol 43(10):1858–1863. https://doi.org/10.3899/jrheum.151220
Zhong L, Pope M, Shen Y, Hernandez JJ, Wu L (2019) Prevalence and incidence of systemic sclerosis: a systematic review and meta-analysis. Int J Rheum Dis 22(12):2096–2107. https://doi.org/10.1111/1756-185X.13716
Moosavi M-S, Barati H (2018) Salivary gland performance in autoimmune diseases: review and meta-analysis. Acta Clin Belg 75(1):19–25. https://doi.org/10.1080/17843286.2018.1540164
Bajraktari IH, Kryeziu A, Sherifi F, Bajraktari H, Lahu A, Bajraktari G (2015) Oral manifestations of systemic sclerosis and correlation with anti-topoisomerase I antibodies (SCL-70). Medical Archives 69(3):153. https://doi.org/10.5455/medarh.2015.69.153-156
Lakota K, Carns M, Podlusky S, Mrak-Poljsak K, Hinchcliff M, Lee J et al (2015) Serum amyloid A is a marker for pulmonary involvement in systemic sclerosis. PLoS ONE 10(1):e0110820
Czirják L, Kumánovics G, Varjú C, Nagy Z, Pákozdi A, Szekanecz Z et al (2008) Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann Rheum Dis 67(1):59–63. https://doi.org/10.1136/ard.2006.066340
Joven BE, Almodovar R, Carmona L, Carreira PE (2010) Survival, causes of death, and risk factors associated with mortality in Spanish systemic sclerosis patients: results from a single university hospital. Semin Arthritis Rheum 39(4):285–293. https://doi.org/10.1016/j.semarthrit.2009.06.002
Minier T, Nagy Z, Bálint Z, Farkas H, Radics J, Kumánovics G et al (2010) Construct validity evaluation of the European Scleroderma Study Group activity index, and investigation of possible new disease activity markers in systemic sclerosis. Rheumatology (Oxford) 49(6):1133–1145. https://doi.org/10.1093/rheumatology/keq022
Hudson M, Steele R, Baron M, Group CSR (2007) Update on indices of disease activity in systemic sclerosis. Semin Arthritis Rheum 37(2):93–98. https://doi.org/10.1016/j.semarthrit.2007.01.005
Scala E, Pallotta S, Frezzolini A, Abeni D, Barbieri C, Sampogna F et al (2004) Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol 138(3):540–546. https://doi.org/10.1111/j.1365-2249.2004.02642.x
Liao R, Yang H-T, Li H, Liu L-X, Li K, Li J-J et al (2022) Recent advances of salivary gland biopsy in Sjögren’s syndrome. Front Med 8:792593. https://doi.org/10.3389/fmed.2021.792593
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed E. Hafez proposed the idea. All authors collected the data. Ahmed E. Hafez, Hany M. Aly, and Abdelhfeez Moshrif designed the study and reviewed the data. AlShaimaa M. Taha performed the statistical analysis, prepared tables, and figures. AlShaimaa M. Taha, Rasha Abdel Noor, and Radwa ElKhouli wrote the manuscript. Ahmed E Hafez and AlShaimaa M. Taha edited the manuscript. All authors reviewed the manuscript and concurred with the findings.
Corresponding author
Ethics declarations
Ethics approval
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hafez, A.E., Taha, A.M., Moshrif, A. et al. Ultrasound abnormalities of the major salivary glands in Egyptian patients with systemic sclerosis. Clin Rheumatol 42, 3351–3360 (2023). https://doi.org/10.1007/s10067-023-06763-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06763-w