Abstract
Introduction
Golimumab, a monoclonal antibody against tumor necrosis factor–α (TNF-α), is used widely for treatment of rheumatic diseases. Long-term persistence is an important factor influencing therapeutic benefit and is a surrogate measure of efficacy. We compared five-year golimumab treatment persistence across studies, indications, and lines of therapy using pooled data from pivotal golimumab Phase III clinical trials.
Methods
This post-hoc analysis evaluated use of golimumab administered subcutaneously (50 or 100 mg every four weeks) for up to five years in 2228 adult participants with rheumatoid arthritis (RA; GO-BEFORE, GO-AFTER, and GO-FORWARD studies), psoriatic arthritis (PsA; GO-REVEAL study), or ankylosing spondylitis (AS; GO-RAISE study). Retention rate differences were evaluated by study, indication, and line of therapy using log-rank tests, and probability of treatment persistence was estimated by Kaplan–Meier analysis.
Results
Golimumab retention rates at Year 5 were consistently high when used as 1st-line therapy (69.8%) and did not differ significantly across the three indications tested (p = 0.5106) or across 1st-line studies (p = 0.2327). Retention at Year 5 was better in participants using golimumab as 1st-line than in those using it as 2nd-line (41.6%) therapy. Participants on 2nd-line golimumab therapy had a longer disease duration (median 9.2 years versus 3.7 years) than those on 1st-line golimumab therapy.
Conclusions
These data support the value of long-term golimumab therapy in patients with chronic, immune-mediated rheumatic diseases when used as 1st-line (RA, PsA, AS) or 2nd-line (RA) therapy.
Key Points • Golimumab is a human monoclonal antibody directed against tumor necrosis factor–α (TNF-α) and is approved widely for the treatment of rheumatic autoimmune diseases. • We compared the probability of treatment persistence, or the time of continuous drug use, for golimumab across five Phase III studies spanning multiple rheumatic indications over five years. • Treatment persistence was favorable and did not differ significantly for participants with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, but persistence was greater when golimumab was used as 1st-line than as 2nd-line biologic therapy. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous therapeutic treatment is important in the context of chronic autoimmune diseases. Treatment persistence, or the total time from initiation to discontinuation of therapy, depends upon drug efficacy, patient satisfaction, safety, and tolerability and is therefore regarded as a useful measure of overall drug effectiveness [1,2,3,4]. Moreover, suboptimal persistence tends to accompany greater patient morbidity and mortality [5], higher disease activity scores [6], and increased utilization of healthcare resources at greater cost [1, 7, 8]. Characterizing drug retention and discontinuation in different patient groups may provide further insight on persistence and, consequently, clinical outcomes [3].
Golimumab is a human monoclonal antibody directed against tumor necrosis factor–α (TNF-α), a potent pro-inflammatory cytokine whose signaling contributes to autoimmune diseases [9,10,11]. Randomized controlled clinical trials have established the efficacy and safety of golimumab treatment in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) [12,13,14,15,16]. Based on these results, golimumab has been approved in multiple countries and regions across the globe for the treatment of several rheumatic disorders. Long-term extensions of these trials reported retention rates of around 70% after five years of follow-up when golimumab was used as a 1st-line TNF-α inhibitor (TNFi) [17,18,19]. Long-term golimumab retention has also been shown to be favorable in several real-world cohorts, most notably in the BIOBADASER registry, which found a 38% retention at years 7 and 8 when golimumab was used as 1st-line therapy [20].
While golimumab retention was described previously in the pivotal Phase III clinical trials (RA: GO-BEFORE [12, 18], GO-AFTER [13, 21], GO-FORWARD [14, 19]; PsA: GO-REVEAL [15, 22]; AS: GO-RAISE [16, 17]), the results of these studies were not compared systematically by Kaplan–Meier analysis, an established standard for the interpretation of drug persistence data. This post-hoc analysis therefore aimed to evaluate golimumab treatment persistence for up to five years using pooled data from these pivotal Phase III studies.
Participants and methods
Study design and data source
Using data collected prospectively from five Phase III, randomized, placebo-controlled clinical trials, this post-hoc analysis evaluated drug retention of golimumab (either 50 mg or 100 mg administered subcutaneously every four weeks) for up to five years in participants with RA, PsA, and AS. Analyses of the five-year data from each of these individual studies have been reported previously [17,18,19, 21, 22].
Drug retention data from four of the five studies (GO-BEFORE, GO-FORWARD, GO-REVEAL, and GO-RAISE) were pooled for analysis of 1st-line golimumab therapy (i.e., treatment with golimumab in participants who had not previously been treated with a TNFi). Data from the remaining study (GO-AFTER) were used for analysis of 2nd- or further line (hereafter, 2nd-line) golimumab therapy (i.e., treatment with golimumab in participants who had previously received and discontinued at least one other TNFi [etanercept, adalimumab, or infliximab] for any reason).
Details of patient eligibility and study design were reported previously [12,13,14,15,16,17,18,19, 21, 22]. Key inclusion and exclusion criteria for each of the five studies are summarized in Supplemental Table 1. The similar eligibility criteria used for the four 1st-line golimumab therapy studies enabled pooled analysis in the present report. Briefly, these studies included adult patients who had an established diagnosis for the indication being studied without any other potentially confounding inflammatory diseases. In all studies except GO-AFTER, patients were naïve to TNFi therapy.GO-BEFORE was the only study to evaluate patients naïve to methotrexate (i.e., had not received more than 3 weekly doses of oral methotrexate for RA at any time).
Statistical analysis
Baseline demographic and disease characteristics were described with summary descriptive statistics. Kaplan–Meier analyses were used to estimate the probability of golimumab persistence over time by study, indication, and line of therapy. Group differences in these analyses were evaluated by the log-rank test. Golimumab persistence rates were determined at baseline (Week 0), Year 1 (Week 52), Year 2 (Week 104), Year 3 (Week 156), Year 4 (Week 208), and Year 5 (Week 252) for all studies. A multivariate Cox regression model was used to analyze predictive factors related to overall golimumab persistence, which was built using the purposeful selection process and stepwise selection method. All analyses were performed using SAS version 9.4. No adjustments for multiplicity were made.
Results
Participant demographic and baseline characteristics are summarized descriptively for the pooled 1st-line RA studies and by line of therapy (1st-line vs 2nd-line) (Table 1). Among the 2228 total participants enrolled in the five trials, 1797 participants received golimumab as 1st-line therapy (RA = 1050; PsA = 394; AS = 353) and 431 participants received golimumab as 2nd-line therapy (all with RA). Compared with the pooled 1st-line golimumab analysis cohort, participants that received 2nd-line golimumab had a longer disease duration (median of 9.2 years versus 3.7 years), were more likely to be female (78.7% versus 62.2%) and older than 50 years (61.5% versus 41.2%). In addition to having used and discontinued other TNFi therapies, more participants in the 2nd-line cohort had used prior corticosteroids (52.7% versus 41.3%) or methotrexate (66.1% versus 58.1%). The disproportionately higher proportion of RA participants in the 1st-line pooled studies (RA represents 58.4% of 1st-line studies) is likely to have influenced some of the observed baseline differences between the 1st- vs 2nd-line studies. Specifically, the higher proportion of females and prior medications (corticosteroids and methotrexate) seen in the pooled RA studies compared to the 1st-line pooled studies and 2nd-line RA study suggest that these observed differences in 2nd-line vs 1st-line therapy may be more indicative of indication as opposed to line of therapy. Supplemental Table 2, which further stratifies the participant baseline characteristics by study, shows the expected differences in gender and prior medications across the individual rheumatic diseases. In contrast, the notably shorter disease duration among participants in the pooled RA 1st-line studies (as well as the 1st-line compared to the 2nd-line RA study) suggest disease duration is more influenced by line of therapy than by indication. The same trend in disease duration was observed for each of the 1st-line rheumatic diseases compared to the 2nd-line RA study (Supplemental Table 2).
Kaplan–Meier analysis was used to directly compare the modeled probability of golimumab treatment persistence over five years for the GO Phase III trials. Retention rates are presented by study, line of therapy, and indication in Table 2. In the pooled 1st-line therapy cohort (GO-BEFORE, GO-FORWARD, GO-REVEAL and GO-RAISE), golimumab retention was high across the five-year duration, with a probability of retention at Year 1 of 87.8% (95% CI: 86.2–89.2) and at Year 5 of 69.8% (95% CI: 67.6–71.9). Long-term treatment retention of 2nd-line golimumab (GO-AFTER) was lower than that of 1st-line golimumab, but remained favorable, with a probability of retention at Year 1 of 76.1% (95% CI: 71.8–79.9) and at Year 5 of 41.6% (95% CI: 36.8–46.3). Among the four individual 1st-line studies, rates were also similar, with retention at Year 1 ranging from 86.4% (GO-BEFORE) to 89.6% (GO-REVEAL) and from 67.0% (GO-BEFORE) to 71.8% (GO-FORWARD and GO-RAISE) at Year 5.
Results from the four 1st-line therapy trials were used to test whether conditions particular to these trials could contribute to differences in persistence at any time point (Fig. 1). Golimumab retention rates were consistent across each of the four 1st-line studies across their five-year durations (log-rank test: p = 0.2327).
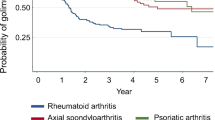
Next, the modeled probability of golimumab treatment persistence was compared across the three rheumatic indications in the 1st-line therapy trials (Fig. 2), which also showed no differences in golimumab retention rates across these indications (log-rank test: p = 0.5106).
In addition, the modeled probability of golimumab treatment persistence of the pooled cohort of participants that received golimumab as 1st-line therapy (GO-BEFORE, GO-FORWARD, GO-REVEAL, and GO-RAISE) was compared with those that received golimumab as 2nd-line therapy (GO-AFTER) (Fig. 3). Treatment retention was higher in participants using golimumab as a 1st-line therapy (pooled indications) than in those using it as 2nd-line therapy (log-rank test: p < 0.0001).
Lastly, the modeled probability of golimumab treatment persistence of the pooled cohort of RA trial participants that received golimumab as 1st-line therapy was compared with that of the RA trial participants that received golimumab as 2nd-line therapy (Fig. 4). Treatment retention was higher in RA trial participants using golimumab as a 1st-line therapy (pooled RA trials) than in those using it as 2nd-line therapy (log-rank test: p < 0.0001).
The evaluation of predictive factors of golimumab treatment retention in the overall study population showed a significant association with race and concomitant methotrexate treatment in addition to indication and line of therapy (Supplemental Table 3). Of note, hazard ratios from the multivariate analysis suggest participants of Black race had a higher risk of not remaining on golimumab treatment compared to participants of Caucasian and Other races (Supplemental Table 4). In contrast, there was a higher likelihood of treatment persistence among participants with AS or PsA compared to RA as well as with AS compared to PsA. Participants receiving concomitant methotrexate also had a higher likelihood of treatment retention compared to those not receiving methotrexate.
Discussion
Treatment persistence, or the total time from initiation to discontinuation of therapy, has been used as a surrogate measure of overall treatment success because it is influenced by drug efficacy, patient satisfaction, safety, and tolerability. The present study compared the treatment persistence of golimumab, a TNFi often taken for years by patients with chronic rheumatic disease, across multiple clinical trials in different rheumatic disease indications and lines of therapy. The results of this post-hoc analysis indicate highly favorable long-term retention of golimumab for trial participants with RA, PsA, or AS, typically around 70% at Year 5 when taken as a 1st-line biologic TNFi. No statistically significant differences in retention rates were observed across either 1st-line clinical trials (p = 0.2327) or disease indications (p = 0.5106). In comparison to the 1st-line trials, long-term golimumab retention was lower for participants with RA receiving 2nd-line therapy in the GO-AFTER trial (p < 0.0001). Similarly, among participants with RA, the golimumab retention rate was lower when used as 2nd-line therapy than when it was used as 1st-line therapy (p < 0.0001). Nonetheless it is noteworthy that over 40% of participants who already discontinued one or more TNFi therapies remained on golimumab at Year 5 in the GO-AFTER trial cohort.
The present study has a couple of important limitations. The disproportionately higher proportion of participants with RA compared to participants with PsA and AS in the 1st-line study cohort skew the overall baseline characteristic profile toward characteristics more typical of an RA patient population. Pooling the three RA 1st-line studies for comparison to the 2nd-line study (all RA) provides a clearer picture of the true differences between 1st- and 2nd-line therapy, highlighting longer disease duration with 2nd-line therapy. While the comparable probability of golimumab retention in each of the individual indications confirmed that it was appropriate to pool the 1st-line therapies for comparison to the 2nd-line therapies, inherent differences in disease characteristics and small subgroup sample sizes limited the evaluation of predictors of persistence to factors collected uniformly across all studies, including demographic and concomitant medications. Moreover, the lack of 2nd-line golimumab data from randomized controlled trials in PsA and AS limits generalizability beyond the RA patient populations.
Higher treatment persistence with 1st-line than with 2nd-line TNFi treatment is consistent with previous studies. Similar findings have been reported with 1st-line compared with 2nd-line golimumab treatment in patients in real-world clinical practice [20]. Lower retention rates are generally expected for patients or clinical trial participants who have already received and discontinued more than one drug in the same class because these individuals tend to have more established disease and are likely to be more difficult to treat [23,24,25]. The predictor analysis in the overall population of the current study showed a higher probability of treatment retention among participants with AS or PsA compared to RA as well as with AS compared to PsA. In addition to indication and line of therapy, the present study identified non-black race and concomitant medication with methotrexate as factors that promote treatment persistence. To improve treatment persistence rates for individuals with recalcitrant rheumatic disease, future studies comparing the specific reasons for discontinuation between 1st- and 2nd-line therapy groups, as well as factors associated with continued drug use particularly in 2nd-line patients, may be warranted. In addition, our data are limited to 2nd-line use of golimumab in participants with RA because this is the only population investigated for golimumab 2nd-line use in a randomized controlled trial. Whether golimumab 2nd-line retention rates differ across individuals with RA, PsA, or AS remains to be further explored, but real-world evidence suggests lower 2nd-line retention with RA [20]. Previous studies suggest that golimumab retention rates are at least as good as or better than those of other TNFi compounds in patients with rheumatic diseases [7, 20, 26,27,28,29].
A recent retrospective analysis of the BIOBADASER database reported rates of golimumab persistence lower than those found in our analysis of the GO Phase III trials [20]. The overall five-year retention rate in that study, including pooled data for approved indications (RA, PsA, and AS) and lines of therapy, was 43.9% (95% CI: 40.2–47.6), and approximately 56% for 1st-line therapy in particular [20]. The 3-to-5-year retention rates reported in other studies for golimumab as 1st or greater line of therapy range from approximately 35% to 65% [29,30,31,32,33,34,35]. Patients in clinical practice can be more difficult to treat and may exhibit more disease comorbidities than the well-controlled clinical trial cohorts, possibly leading to lower retention. Rates observed in clinical trials are therefore not necessarily predictive of real-world drug persistence [3]. Nonetheless, the results from BIOBADASER remarkably showed that approximately 38% of the total adult rheumatology patient population remained on golimumab treatment for eight years after treatment initiation in a real-world setting [20].
Golimumab persistence reported in the literature is variable and is likely due to small sample sizes as well as differences in study designs and study populations (consisting of biologic naïve and biologic experienced, in addition to multiple lines of therapy), making inter study comparisons limited. For example, not all reports are consistent with the current finding that golimumab treatment persistence over 5 years does not differ between the approved rheumatic indications. In the BIOBADASER population, golimumab retention rates were shown to be higher among patients with AS or PsA than those with RA; however, this population consisted of a mixture of therapy lines that were roughly similar across the indications [20, 23]. Similarly, another study in a Spanish cohort reported that more patients with AS and PsA continued their first biologic drug than did patients with RA after a mean 3.8 years of follow-up [36]. A study of 328 individuals with RA, PsA, or AS at four centers in Greece reported overall high golimumab retention (62% with pooled lines of therapy) at three years, but rates were higher for patients with AS than for those with RA or PsA [35]. However, other shorter-term studies in Italy [37] and France [38] reported similar retention rates across indications after two years of golimumab treatment, particularly for 1st-line therapy. While some differences in real-world drug use could be attributed to differences in healthcare [39, 40], factors affecting retention rates in different indications are not yet fully understood and would be an important topic for future investigation, particularly in large cohorts with long follow-up duration.
Conclusion
This post-hoc analysis of data collected prospectively from five Phase III clinical trials of golimumab in participants with rheumatic diseases presents, for the first time, comparative persistence results using Kaplan–Meier analysis. High treatment retention was seen across indications (RA, PsA, and AS), with at least two-thirds of patients estimated to remain on therapy five years after treatment initiation. Probability of long-term golimumab treatment retention with 2nd-line therapy, while lower than that of 1st-line therapy, also remained favorable with approximately 42% of participants estimated to remain on therapy at Year 5. Taken together, the present analysis supports the value of long-term golimumab use as 1st-line therapy in patients with rheumatic diseases (RA, PsA and AS) and as 2nd-line therapy in patients with RA.
Data availability
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
References
Svedbom A, Dalen J, Black CM, Kachroo S (2017) Persistence and costs with subcutaneous TNF-alpha inhibitors in immune-mediated rheumatic disease stratified by treatment line. Patient Prefer Adherence 11:95–106. https://doi.org/10.2147/PPA.S119808
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB (2009) Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15:728–740. https://doi.org/10.18553/jmcp.2009.15.9.728
Bolge SC, Goren A, Tandon N (2015) Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence 9:121–131. https://doi.org/10.2147/PPA.S70834
Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marchesoni A, Meroni PL (2016) Twelve-Year Retention Rate of First-Line Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: Real-Life Data From a Local Registry. Arthritis Care Res (Hoboken) 68:432–439. https://doi.org/10.1002/acr.22788
Maniadakis N, Toth E, Schiff M, Wang X, Nassim M, Szegvari B, Mountian I, Curtis JR (2018) A Targeted Literature Review Examining Biologic Therapy Compliance and Persistence in Chronic Inflammatory Diseases to Identify the Associated Unmet Needs, Driving Factors, and Consequences. Adv Ther 35:1333–1355. https://doi.org/10.1007/s12325-018-0759-0
Scire CA, Caporali R, Sarzi-Puttini P, Frediani B, Di Franco M, Tincani A, Sinigaglia L, Sfriso P, Tirri R, Bellis E et al (2013) Drug survival of the first course of anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: analysis from the MonitorNet database. Clin Exp Rheumatol 31:857–863
Dalen J, Svedbom A, Black CM, Lyu R, Ding Q, Sajjan S, Sazonov V, Kachroo S (2016) Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int 36:987–995. https://doi.org/10.1007/s00296-016-3423-5
Harnett J, Wiederkehr D, Gerber R, Gruben D, Koenig A, Bourret J (2016) Real-world evaluation of TNF-inhibitor utilization in rheumatoid arthritis. J Med Econ 19:91–102. https://doi.org/10.3111/13696998.2015.1099538
Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP (2008) Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 117:244–279. https://doi.org/10.1016/j.pharmthera.2007.10.001
Mazumdar S, Greenwald D (2009) Golimumab MAbs 1:422–431. https://doi.org/10.4161/mabs.1.5.9286
Rossini M, Viapiana O, Orsolini G, Fracassi E, Idolazzi L, Gatti D, Adami S, Govoni M (2015) Why golimumab in the treatment of psoriatic arthritis, ankylosing spondylitis and rheumatoid arthritis? Reumatismo 66:285–303. https://doi.org/10.4081/reumatismo.2014.799
Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, Nash P, Amante EJ, Churchill M, Park W et al (2009) Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 60:2272–2283. https://doi.org/10.1002/art.24638
Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, Gaylis N, Murphy FT, Neal JS, Zhou Y et al (2009) Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. The Lancet 374:210–221. https://doi.org/10.1016/s0140-6736(09)60506-7
Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, Pazdur J, Bae SC, Palmer W, Zrubek J et al (2009) Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 68:789–796. https://doi.org/10.1136/ard.2008.099010
Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M et al (2009) Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 60:976–986. https://doi.org/10.1002/art.24403
Inman RD, Davis JC Jr, Heijde D, Diekman L, Sieper J, Kim SI, Mack M, Han J, Visvanathan S, Xu Z et al (2008) Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 58:3402–3412. https://doi.org/10.1002/art.23969
Deodhar A, Braun J, Inman RD, van der Heijde D, Zhou Y, Xu S, Han C, Hsu B (2015) Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis 74:757–761. https://doi.org/10.1136/annrheumdis-2014-205862
Emery P, Fleischmann RM, Strusberg I, Durez P, Nash P, Amante EJ, Churchill M, Park W, Pons-Estel B, Han C et al (2016) Efficacy and Safety of Subcutaneous Golimumab in Methotrexate-Naive Patients With Rheumatoid Arthritis: Five-Year Results of a Randomized Clinical Trial. Arthritis Care Res (Hoboken) 68:744–752. https://doi.org/10.1002/acr.22759
Keystone EC, Genovese MC, Hall S, Bae SC, Han C, Gathany TA, Xu S, Zhou Y, Leu JH, Hsia EC (2016) Safety and Efficacy of Subcutaneous Golimumab in Patients with Active Rheumatoid Arthritis despite Methotrexate Therapy: Final 5-year Results of the GO-FORWARD Trial. J Rheumatol 43:298–306. https://doi.org/10.3899/jrheum.150712
Pombo-Suarez M, Seoane-Mato D, Diaz-Gonzalez F, Cea-Calvo L, Sanchez-Alonso F, Sanchez-Jareno M, Jovani V, Garcia-Magallon B, Martinez-Gonzalez O, Campos-Fernandez C et al (2022) Long-term retention of golimumab treatment in clinical practice in a large cohort of patients with rheumatoid arthritis, axial spondyloarthritis and psoriatic arthritis. Musculoskeletal Care. https://doi.org/10.1002/msc.1684
Smolen JS, Kay J, Doyle M, Landewe R, Matteson EL, Gaylis N, Wollenhaupt J, Murphy FT, Xu S, Zhou Y, Hsia EC (2015) Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther 17:14. https://doi.org/10.1186/s13075-015-0516-6
Kavanaugh A, McInnes IB, Mease P, Krueger GG, Gladman D, van der Heijde D, Zhou Y, Lu J, Leu JH, Goldstein N, Beutler A (2014) Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 73:1689–1694. https://doi.org/10.1136/annrheumdis-2013-204902
Pombo-Suarez M, Sanchez-Piedra C, Garcia-Magallon B, Perez-Gomez A, Manrique-Arija S, Martin-Domenech R, Colazo M, Campos C, Campos J, Del Pino-Montes J et al (2021) Factors associated with long-term retention of treatment with golimumab in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis: an analysis of the Spanish BIOBADASER registry. Clin Rheumatol 40:3979–3988. https://doi.org/10.1007/s10067-021-05742-3
Hernandez MV, Sanchez-Piedra C, Garcia-Magallon B, Cuende E, Manero J, Campos-Fernandez C, Martin-Domenech R, Del Pino-Montes J, Manrique S, Castro-Villegas MC et al (2019) Factors associated with long-term retention of treatment with golimumab in a real-world setting: an analysis of the Spanish BIOBADASER registry. Rheumatol Int 39:509–515. https://doi.org/10.1007/s00296-018-4177-z
Mutoh T, Nagai T, Shirai T, Okazaki S, Sato H, Fujii H (2022) Predictive factors for retention of golimumab over a median 4-year duration in Japanese patients with rheumatoid arthritis in a real-world setting: A retrospective study and literature review. Int J Rheum Dis 25:335–343. https://doi.org/10.1111/1756-185X.14281
Ebina K, Hirano T, Maeda Y, Yamamoto W, Hashimoto M, Murata K, Takeuchi T, Shiba H, Son Y, Amuro H et al (2020) Drug retention of 7 biologics and tofacitinib in biologics-naive and biologics-switched patients with rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 22:142. https://doi.org/10.1186/s13075-020-02232-w
Egeberg A, Roseno NAL, Aagaard D, Lorup EH, Nielsen ML, Nymand L, Kristensen LE, Thyssen JP, Thomsen SF, Cordtz RL et al (2022) Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis - A nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum 53:151979. https://doi.org/10.1016/j.semarthrit.2022.151979
Kim HA, Lee SK, Oh S, Park EH, Park YB, Shin K (2021) Comparison of Retention Rates Between Tumor Necrosis Factor-alpha Inhibitors in Patients With Ankylosing Spondylitis: Data From the Korean College of Rheumatology Biologics Registry. Front Med (Lausanne) 8:689609. https://doi.org/10.3389/fmed.2021.689609
Svedbom A, Storck C, Kachroo S, Govoni M, Khalifa A (2017) Persistence with golimumab in immune-mediated rheumatic diseases: a systematic review of real-world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Patient Prefer Adherence 11:719–729. https://doi.org/10.2147/PPA.S128665
Aaltonen KJ, Joensuu JT, Pirila L, Kauppi M, Uutela T, Varjolahti-Lehtinen T, Yli-Kerttula T, Isomaki P, Nordstrom D, Sokka T (2017) Drug survival on tumour necrosis factor inhibitors in patients with rheumatoid arthritis in Finland. Scand J Rheumatol 46:359–363. https://doi.org/10.1080/03009742.2016.1234641
Alegre-Sancho JJ, Juanola X, Rodriguez-Heredia JM, Manero J, Villa-Blanco I, Laiz A, Arteaga MJ, Cea-Calvo L, Gonzalez CM (2021) Effectiveness and persistence of golimumab as a second biological drug in patients with spondyloarthritis: A retrospective study. Medicine (Baltimore) 100:e25223. https://doi.org/10.1097/MD.0000000000025223
Chimenti MS, Conigliaro P, Caso F, Costa L, Ortolan A, Triggianese P, Tasso M, Fonti GL, Lorenzin MG, Perricone R, Ramonda R (2022) Long-term effectiveness and drug survival of golimumab in patients affected by psoriatic arthritis with cutaneous involvement. Clin Rheumatol 41:75–84. https://doi.org/10.1007/s10067-021-05874-6
Michelsen B, Sexton J, Wierod A, Bakland G, Rodevand E, Kroll F, Kvien TK (2020) Four-year follow-up of inflammatory arthropathy patients treated with golimumab: Data from the observational multicentre NOR-DMARD study. Semin Arthritis Rheum 50:12–16. https://doi.org/10.1016/j.semarthrit.2019.07.003
Serrano-Benavente B, Valor L, Del Rio Blasco T, Janta I, Gonzalez Benitez R, Nieto-Gonzalez JC, Martinez-Barrio J, Ovalles Bonilla JG, Ariza A, Lopez-Longo FJ et al (2022) Long-Term Retention Rate of Golimumab in Patients With Rheumatoid Arthritis, Psoriatic Arthritis, and Spondyloarthritis in a Real-Life Setting. J Clin Rheumatol 28:e150–e155. https://doi.org/10.1097/RHU.0000000000001695
Thomas K, Flouri I, Repa A, Fragiadaki K, Sfikakis PP, Koutsianas C, Kaltsonoudis E, Voulgari PV, Drosos AA, Petrikkou E et al (2018) High 3-year golimumab survival in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: real world data from 328 patients. Clin Exp Rheumatol 36:254–262
Canete JD, Naranjo A, Calvo J, Ordas C, Aragon B, Nocea G, Roset M, Fernandez-Nebro A (2020) Biological Treatment Patterns in Patients with Inflammatory Joint Diseases. Retrospective Study with 4 Years Follow-up. Reumatol Clin (Engl Ed) 16:447–454. https://doi.org/10.1016/j.reuma.2018.11.007
Iannone F, Favalli EG, Caporali R, D’Angelo S, Cantatore FP, Sarzi-Puttini P, Foti R, Conti F, Carletto A, Gremese E et al (2021) Golimumab effectiveness in biologic inadequate responding patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis in real-life from the Italian registry GISEA. Joint Bone Spine 88:105062. https://doi.org/10.1016/j.jbspin.2020.07.011
Flipo RM, Tubach F, Goupille P, Lespessailles E, Harid N, Sequeira S, Bertin P, Fautrel B (2021) Real-life persistence of golimumab in patients with chronic inflammatory rheumatic diseases: results of the 2-year observational GO-PRACTICE study. Clin Exp Rheumatol 39:537–545. https://doi.org/10.55563/clinexprheumatol/zizo0l
Dalen J, Luttropp K, Svedbom A, Black CM, Kachroo S (2020) Healthcare-Related Costs Associated with Switching Subcutaneous Tumor Necrosis Factor-alpha Inhibitor in the Treatment of Inflammatory Arthritis: a Retrospective Study. Adv Ther 37:3746–3760. https://doi.org/10.1007/s12325-020-01425-8
Wolf D, Skup M, Yang H, Fang AP, Kageleiry A, Chao J, Mittal M, Lebwohl M (2017) Clinical Outcomes Associated with Switching or Discontinuation from Anti-TNF Inhibitors for Nonmedical Reasons. Clin Ther 39:849-862 e846. https://doi.org/10.1016/j.clinthera.2017.03.005
Acknowledgements
The authors would like to thank Jennifer Rotonda and Michele McColgan for assistance with preparing the manuscript for submission. JR and MM are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in Merck & Co., Inc., Rahway, NJ, USA.
Funding
The studies included in this analysis were funded by Janssen Research & Development, LLC (Spring House, PA, USA) and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, New Jersey, USA.
Author information
Authors and Affiliations
Contributions
As per ICMJE rules, all authors contributed to the conception, design or planning; acquisition/analysis of the data; and/or interpretation of the results. They also drafted the manuscript and/or critically reviewed the manuscript for important intellectual content.
All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
All studies were approved by the appropriate ethics committee and performed in accordance with the ethical standards described in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the studies analyzed in this report.
Disclosures
CLJW, AGM, JL, SDB, and MG are current or former employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in Merck & Co., Inc., Rahway, NJ, USA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weinstein, C.L.J., Meehan, A.G., Lin, J. et al. Long-term golimumab persistence: Five-year treatment retention data pooled from pivotal Phase III clinical trials in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Clin Rheumatol 42, 3397–3405 (2023). https://doi.org/10.1007/s10067-023-06760-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06760-z