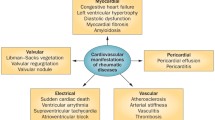
Abstract
Cardiovascular involvement in juvenile rheumatic diseases is the primary manifestation in paediatric vasculitis and a major organ manifestation in paediatric connective tissue diseases. Though coronary vasculitis is the prototypical manifestation of Kawasaki disease, it can also be seen in patients with polyarteritis nodosa. Pericarditis is the most common manifestation seen in juvenile rheumatic diseases like systemic onset JIA, and lupus. Cardiac tamponade, valvular insufficiency, aortic root dilatation and arrhythmias are seen rarely. Cardiac involvement is often recognized late in children. The development of cardiac disease in juvenile systemic sclerosis is associated with a poor outcome. In long term, childhood onset of rheumatic diseases predisposes to diastolic dysfunction and premature atherosclerosis during adulthood.
Key Points • Pericarditis is the most common cardiac manifestation in SLE and can lead to tamponade. • Conduction defects are common in juvenile mixed connective tissue disease and systemic sclerosis. • Pulmonary hypertension is a significant contributor to mortality in juvenile systemic sclerosis. • In Kawasaki disease, early treatment can reduce risk of coronary artery aneurysms. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac disease in paediatric rheumatic diseases is not uncommon but is less recognized. It can present as pericarditis, myocarditis, valvulitis, conduction defects, coronary vasculitis and even as nonbacterial endocarditis [1, 2]. It can also be secondary to hypertension in diseases like in Takayasu arteritis or systemic lupus erythematosus [3, 4]. Cardiac involvement is common in Kawasaki disease and acute rheumatic fever. In a large majority of patients with SLE, juvenile arthritis and systemic sclerosis it can be subclinical. Cardiac involvement is associated with higher morbidity and mortality especially in children with juvenile systemic sclerosis [5].
With the advent of better non-invasive techniques like spectral echocardiography, coronary angiography using contrast CT, cardiac MRI, subclinical disease can be easily recognized [6]. Studies in paediatric rheumatic diseases using carotid intima media thickness has led to recognition of premature atherosclerosis in children [7, 8]. This can increase the CV mortality in adulthood. In SLE, onset in childhood was an independent risk factor for mortality even after adjusting for duration of disease [9]. This emphasizes the importance of screening for cardiac diseases as well as systematic follow up of these patients for modifying their disease course. This narrative review analyses the different cardiac manifestations of childhood autoimmune disease, systemic vasculitis, autoinflammatory diseases and juvenile idiopathic arthritis and their short term as well as long term cardiovascular morbidity.
A PubMed search was done for relevant articles in English language on 2/12/22. A search strategy for article titles using the keywords (("pediatric"[Title] OR "juvenile"[Title] OR "child"[Title] OR "children"[Title]) AND ("cardiovascular"[Title] OR "cardiac"[Title])) AND ("rheumatological"[Title]) was done. Similarly, search was done substituting “rheumatological” with “autoimmune”, “arthritis”, “vasculitis”, “connective tissue disease”, “systemic lupus erythematosus”, “systemic sclerosis”, “dermatomyositis”, “Takayasu” and “Kawasaki”. Search for “autoinflammatory diseases” and “neonatal lupus” was made independently using these key words. Cross reference from relevant articles was done. A narrative review was written in accordance with previously published articles on this format [10].
Autoimmune diseases
Juvenile systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a multisystem disease associated with an array of autoantibodies. Its prevalence and incidence in children is estimated to be 1.8—25.7 and 0.3 – 2.5 per 10,000 [11]. Juvenile SLE is more severe and has higher prevalence of severe major organ manifestation compared to adults [12, 13]. Though renal and central nervous system manifestations are more common, cardiac involvement in the form of pericarditis and myocarditis is also fourfold higher in children compared to adults [14].
The frequency of cardiac disease varies from 17–42% and it can involve pericardium, myocardium, valves and the coronary arteries (Table 1) [14-17]. The timing of cardiac involvement in juvenile SLE is variable. Initial cohorts described that the frequency of cardiopulmonary involvement progressively increased from 15 to 30% from 2 to 5 years after onset of disease. More recent cohorts have suggested that cardiac abnormalities are evident at the time of diagnosis in 84% [15]. This may reflect better screening tools and techniques. Further, involvement of the heart may predate the diagnosis of SLE in 19%, with the highest frequency within the first year of diagnosis [14, 18].
African American race, renal and neurolupus are associated with increased incidence of cardiac involvement, while Asians and females have lower incidence [14]. Anti-Ro and anti-La antibodies are associated with cardiac involvement, while no such association has been seen with anti-Sm or anti-RNP antibodies [18]. Though there is limited data available on its pathology, presence of immunoglobulin and complement deposits in the perivascular tissue and pericardium suggests an immune complex mediated tissue damage [43]. Valvular pathology includes: valve thickening and less commonly verrucous endocarditis. Fibrin clumps, focal necrosis and mononuclear infiltrates are seen in active lesions while fibrovascular tissue with calcification is a feature of long-standing valvular disease.
Pericardial disease is the most common form of cardiac disease in juvenile SLE. Though acute pericarditis occurs in 10% of children in large case series, pericardial effusion has been described in to 26%—33% of children in smaller series (Fig. 1) [14, 19]. Unlike adults, large pericardial effusions and tamponade is more common in children. In a small proportion, it can result in haemodynamic instability [14, 15]. At the same time, it is not uncommon to see clinically silent cardiac disease [16, 20].
Myocarditis is less common and is reported in 1—16% of children with SLE [14, 18, 21]. In an echocardiographic study left ventricular ejection fraction was reduced in 13% of patients with SLE [15]. In another retrospective study, children with high disease activity and vasculitis had worse diastolic dysfunction. Significantly, this study assessed patients within days of diagnosis, thus avoiding impact of drugs and hypertension on the myocardial function [19].
Echocardiographic evaluation in adults SLE patients found valvular regurgitation in 25%, valve thickening in 51% and vegetations in 43% of patients [44]. However, in children valvular insufficiency was reported in 6–16% with 6% having severe valvular dysfunction [14, 15, 17, 19]. Valvular dysfunction has been variably associated with presence of antiphospholipid antibodies [45, 46]. Vegetations often resolve over the course of the disease, while valvular insufficiency has variable outcome [44].
Subclinical atherosclerosis as measured by carotid pulse wave velocity, augmentation index and carotid intima media thickness are higher in paediatric lupus patients compared to controls [7]. The APPLE cohort observed that, apart from traditional risk factors, increasing age, longer SLE duration, increased creatinine clearance and azathioprine use was associated with increased CIMT. The association with prednisolone was non-linear with moderate doses being associated with reduced CIMT, while low and high doses were associated with increased CIMT [22].
Pericarditis can be managed with Non-steroidal anti-inflammatory drugs but presence of significant effusion or myocarditis warrants use of corticosteroids. Pulse corticosteroids may be needed in patients with severe myocarditis Libman-Sacks endocarditis warrants high dose immunosuppression [1]. Cardiovascular involvement in juvenile lupus increases the risk of mortality [15]. Infective endocarditis may occur in conjunction with Libman-Sack vegetations, and generally is associated with increased mortality and morbidity in SLE patients [44]. As children are living longer with SLE, subclinical atherosclerosis can have long term consequences thus all attempts should be made to reduce the risk.
Neonatal lupus
Neonatal lupus erythematosus accounts for 95–99% of congenital atrioventricular heart block (CAHB) diagnosed before 6 months of age. Most of the mothers of these neonates have anti-Ro or anti-La antibodies. CAHB results from transplacental transfer of maternal antibodies to the foetus, leading to poor clearance of apoptotic cardiocytes. This results in inflammation and fibrosis of the conduction system thus leading to heart block.
Though only 2% of mothers with anti-Ro antibodies have babies with CAHB, the risk increases in subsequent pregnancy to 20% suggesting presence of additional risk factors in susceptible mothers. Regular monitoring of the foetus between 16–24 weeks of gestation is recommended for early recognition. In one series, 54% of the prenatally diagnosed CAHB babies born alive required pacemaker in the neonatal period and nearly 70% eventually requiring permanent pacemakers at some time point. When CAHB was diagnosed in the neonatal period, permanent pacemaker was required in 73% by a median age of 9 years. Almost a quarter of babies diagnosed prenatally with CAHB die in neonatal period [47]. Use of prophylactic hydroxychloroquine in mothers has reduced the risk of subsequent CAHB in next baby [48].
Juvenile dermatomyositis
The prevalence of cardiac abnormalities in juvenile dermatomyositis (JDM) is estimated to be 2.9% [49]. This is lower than the estimate of 9–72% in adults with inflammatory myositis [50]. Histopathology of cardiac tissue in inflammatory myositis is characterised by lymphocytic infiltration and fibrosis of the conduction system and interstitial inflammatory infiltrate, contraction band necrosis and patchy focal fibrosis of myocardium. The vasculature shows active vasculitis with intimal hyperplasia [51]. Active myocarditis characterised by diffuse interstitial and perivascular infiltrate is seen in 30% of autopsy specimens [52]. Medial smooth muscle hyperplasia with or without intimal hyperplasia are features of small vessel disease.
Children predominantly have subclinical cardiac disease (Table 1). Echocardiographic evaluation has identified cardiac abnormalities in 25% cases, pericardial effusion being the most common followed by valvular regurgitation, albeit all being of mild severity [23]. Based on follow up studies, pericarditis is seen in 7%, and mitral and aortic regurgitation are seen in 3% each. Hypertension was seen at the disease onset in 11.8% [24]. In a study evaluating mortality in JDM patients, pulmonary hypertension was present in 2, myocarditis in 1 and acute and chronic cardiac failure in 2 of 17 deaths. Electrocardiographic changes including conduction blocks and QTc prolongation have been described in 9–50% cases [23]. Similar to structural changes, rhythm abnormalities are also higher in adults compared with JDM [24].
Long term follow-up of JDM patients for more than 15 years found left ventricular diastolic or systolic dysfunction in 18% of patients. The same group showed that JDM patients also have higher frequency of diastolic dysfunction as compared to age matched adults [24]. When compared to those with only pulmonary involvement, JDM children with cardiac disease on follow-up had higher disease activity and higher cumulative prednisolone dose [25]. Two- dimensional speckle training echocardiography, cardiac MRI and technetium pyrophosphate scintigraphy are new techniques that have improved sensitivity for detecting subclinical myocardial dysfunction in JDM [53]. While cardiac involvement in adults is associated with significant morbidity, a study evaluating predictors of mortality did not find a specific risk associated with cardiac disease in JDM [54]. In a systematic review in adults, cardiac disease contributed to 46% of mortality [50]. In contrast in children it contributes to only 15% of deaths in JDM [54].
Juvenile systemic sclerosis
Juvenile systemic sclerosis (Juvenile SSc) accounts for approximately 1.2%-10% of overall systemic sclerosis cases [5, 26, 27]. The prevalence of cardiac diseases is 6–17% compared to 15–20% in adults [5, 28]. Pathological features include diffuse patchy fibrosis with contraction band necrosis of the myocardium. Intramural coronary arteries show concentric intimal hypertrophy and fibrinoid necrosis while epicardial coronaries are relatively preserved.
Cardiac involvement in juvenile SSc is more common in the limited cutaneous (lcSSc) subset (Table 1) [29]. At the same time, lcSSc is less common in children [26, 27]. Arrhythmias are the most common cardiac feature of Juvenile SSc. Supraventricular extrasystoles and conduction blocks especially right bundle branch blocks are most common. The frequency can vary between 5–47% [29-33, 55]. They are usually asymptomatic but can rarely result in cardiac failure and death [30, 32]. The frequency of conduction abnormalities decrease with age [30]. Pulmonary hypertension is seen in 5–8.6% of children with SSc which is lower compared to the adults [26, 29, 31, 55]. Similar to adults, it is a significant contributor to mortality. Mitral and tricuspid valve regurgitation is rarely present [29, 30]. Cardiac failure is present in 7% [31]. The presence of cardiac abnormalities is not only restricted to systemic disease but is also seen in localised scleroderma with sinus tachycardia present in 53% children [30].
While juvenile SSc has a better survival outcome compared to adults, cardiac involvement in children portends a bad prognosis [5, 56]. Heart involvement, especially cardiac failure and pulmonary hypertension, accounts for 15–66% mortality in children [5, 29, 31, 34-37]. In a multicentre series that included 134 JSSC, cardiac involvement accounted for all deaths in the first year after diagnosis [56]. In a survival analysis, the presence of pericarditis, heart failure or arrhythmias at the time of diagnosis was associated with an increased risk of mortality, and the presence of pulmonary hypertension during the course of disease was associated with an increased risk of mortality. Only pericarditis remained significant after multivariate analysis (OR – 41) [56].
The predominant contribution of myocardial dysfunction to mortality has paved way for improved techniques of echocardiography to assess ventricular function. Speckle tracking echocardiography (STE) has increased sensitivity for myocardial dysfunction compared to conventional assessment of ejection fraction in juvenile SSc [6]. The postulated mechanism is the enhanced ability of Global longitudinal strain (GLS) by STE to detect subendocardial layers affected by microvascular ischemia. Further it has been seen that right ventricular longitudinal strain (RVLS), which is lower in juvenile SSc compared to controls, has a negative correlation with the modified Rodnan skin score (mRSS). Advent of improved screening of cardiac function in juvenile SSc may help guide immunosuppressive and anti-fibrotic therapy.
Juvenile mixed connective tissue disease (Juvenile MCTD)
Juvenile MCTD accounts for 23% of mixed connective tissue diseases. Cardiac involvement accounts for 20% mortality in mixed connective tissue disease [57]. In children with MCTD, cardiovascular involvement is seen in 5% [38]. Myocardial and pericardial disease is more common in children. In a series of 14 children, 4 developed myocarditis and cardiac failure [39]. Pericarditis is present in 16% to 42% [39-41]. Histopathology shows patchy myocardial necrosis, interstitial inflammatory infiltrate and intimal thickening of vessels [39]. Pulmonary artery hypertension is present in 3%—14%[39, 42]. This is less than estimates in adults [58]. Similar to adults, pulmonary hypertension is directly or indirectly related to mortality [58]. Conduction abnormalities have been noted in up to 8% [41].
On assessment of cardiac function 16 years after diagnosis of disease, those with juvenile onset MCTD had lower left ventricular systolic function and right ventricular function compared to age matched healthy controls [59] Similar to other autoimmune diseases, subclinical atherosclerosis is increased in adults with childhood onset MCTD. In a study of 49 cases, the carotid IMT was significantly higher than controls, and correlated with disease duration as well as physician global assessment [60].
Autoinflammatory diseases
Autoinflammatory diseases are a group of diseases that occur due to activation of innate immune system resulting from monogenic defects. Recurrent pericarditis which may be refractory to treatment, cardiac amyloidosis and atherosclerosis are common manifestations of periodic fever syndromes. Pericarditis is seen in Familial Mediterranean Fever (FMF) and TNF receptor 1 associated periodic fever syndrome (TRAPS), while myocarditis is a rarely seen in FMF. Cardiomyopathy has been described in interferonopathies like Aicardi-Goutières syndrome [61]. A recent review has discussed the common cardiac features of periodic fever syndromes [62].
Vasculitis
Cardiac involvement is common in paediatric vasculitis like Kawasaki disease and Takayasu arteritis while it is rare in other systemic vasculitis like IgA vasculitis [63].
Kawasaki disease
Kawasaki disease (KD) is a medium vessel vasculitis that presents as an acute febrile illness in children. The characteristic involvement of coronary arteries makes it important to recognise this condition early. Recent survey based epidemiological data suggests that cardiac sequelae are seen in 2.6% of patients [64]. Coronary artery vasculitis in KD is an acute necrotising arteritis which can progress to a chronic stage characterised by luminal myofibroblastic proliferation. The destruction of the internal elastic lamina and media results in ectasia of the vessel and aneurysm formation. The abnormal luminal flow further predisposes to arterial thrombosis and myocardial ischemia. The combination of luminal myofibroblast proliferation and organised thrombus may result in normalisation of calibre of the coronary lumen even though the primary coronary wall pathology persists.
In large series of more than 500 cases of KD in the pre-intravenous immunoglobulin era (IVIG), coronary angiography and echocardiography when done at 1 month showed coronary artery aneurysms in 24% and 26% respectively [65, 66]. The prevalence reduces to 5 – 14% with IVIG [66, 67, 68]. Epidemiological data from the last two decades suggests that the prevalence of coronary artery dilatations varies between 8.5–16.4% and coronary artery aneurysms 1.2–7.4% [69].
Coronary artery aneurysms predispose to the development of rupture and thrombosis. While both are catastrophic, the former is rare. The coronary artery Z score is based on adjusting coronary artery luminal dimensions for body surface area. Classification based on Z score are as follows: Z score < 2 – no dilatation, Z score 2 – 2.5 – dilatation, Z score ≥ 2.5 – 5 – small aneurysm, Z score ≥ 5 – 10 or absolute dimension < 8 mm – medium aneurysm, Z score ≥ 10 or absolute dimension ≥ 8 mm – large aneurysm. The more the size of aneurysms, the more the risk of thrombosis. This is the basis for close follow up of patients with coronary aneurysms to identify those with an increased risk of thrombosis and thus the group in which thromboprophylaxis must be initiated. Aneurysms of size ≥ 8 mm or a Z score ≥ 10 are predisposed to thrombosis. Development of such ‘giant’ aneurysms warrant the use of anticoagulants in addition to antiplatelet therapy. Myocardial infarction occurs in 1.5 – 1.8% of cases with majority occurring in those with giant aneurysms [65, 70].
Myocardial inflammation is common in KD and is seen in the acute stage. Though biopsy shows abnormalities in all patients early in the course, echocardiographic evidence of left ventricular dysfunction is seen in only 20 – 56% [71, 72]. Unlike necrotising myocarditis, it has a good response to anti-inflammatory medications [73]. Valvular involvement is commonly seen in the acute stage [64]. Cardiac murmurs may be audible in only 1% with mitral regurgitation murmur accounting for majority [70]. Mild mitral regurgitation is present in 27–47% at initial evaluation on echocardiography [72, 74]. However, rarely it may be severe enough to produce cardiac failure [70]. It often resolves on follow up. Aortic regurgitation is described in 4 – 8% cases [72, 75]. The incidence of coronary lesions is higher in patients with valvular pathology [70, 72]. The development of shock in patients with KD (Kawasaki disease shock syndrome) is an indicator of significant cardiac involvement. In a series of 187 children with KD, 7% had Kawasaki disease shock syndrome. Children with shock had a higher percentage of coronary artery abnormalities (62% vs 23%), mitral regurgitation (39% vs 2%) and impaired left ventricular function [76].
Intravenous immunoglobulin (IVIG) remains the mainstay of treatment in KD. The current recommendation is to give IVIG as a single dose over 10 – 12 h in combination with aspirin [77]. Other drugs that have been used with variable success in KD are TNF inhibitors (Infliximab, etanercept), cyclosporine, anakinra and cyclophosphamide [78-82].
In uncomplicated coronary aneurysms, echocardiographic screening at 1 – 2 weeks and then at 6 weeks is recommended. Another group that may not require follow up are those who have mild dilatation (Z score 2 to < 2.5) which decreases to Z score < 2 at 4 – 6 weeks. For those with Z score ≥ 2.5, biweekly evaluation should be done until luminal progression has halted and then weekly. After 6 weeks, screening is determined by the size of the aneurysms, with larger and giant aneurysms, requiring monthly follow up till 3 months [78].
Multisystem inflammatory disease-children
Multisystem inflammatory disease in children (MIS-c) is a hyperinflammatory state seen in children with recent infection or family exposure to SARS-CoV2. The syndrome mimics atypical Kawasaki disease, Kawasaki Disease shock syndrome or Toxic shock syndrome [80]. A recent review concluded that 35% to 100% have left ventricular dysfunction. Coronary artery aneurysms are present in 6% to 24% with majority having mild aneurysms (Z score 2 – 2.5). Arrhythmias are observed in 7% to 60% [81]. Pericarditis is seen in 5.8% and myocarditis in 25.3% [82].
Takayasu arteritis
Takayasu arteritis is a common primary vasculitis in children with a median age of onset of 12 to 14 years [3, 4, 83, 84]. It constitutes 14% to 32% of all Takayasu arteritis cases [3, 4, 85, 86]. Cardiac involvement is present in 40% of Takayasu arteritis [85]. Takayasu arteritis is a granulomatous pan-arteritis with characteristic involvement of elastic arteries. Histopathological features in the heart include biventricular hypertrophy, predominantly left ventricular, secondary to hypertension and aortic and mitral regurgitation [87]. Features of myocarditis like mononuclear cell infiltration and necrosis of myofibers are also seen.
Paediatric Takayasu arteritis differs in its presentation compared to adults. Fever and systemic inflammation are more marked while claudication and bruits are less common. The increased occurrence of this ‘pre-pulseless phase’ delays the diagnosis of Takayasu arteritis in children [4, 83, 85]. The delayed diagnosis often results in children presenting acutely with cardiac failure. In a series of 107 patients, 67% of cases below 15 years of age presented for the first time as acute cardiac failure [88]. Other series have shown lower rates of cardiac failure of 14 – 66% [4, 89, 90]. Myocarditis has mainly been described based on endomyocardial biopsy and autopsy with paucity from echocardiographic studies [91, 92]. Pericardial effusion is present in 4.3%—12% [4, 84, 86].
Hypertension is seen in two third cases and is the most common presentation in children in more recent series [3]. Cardiomyopathy is seen in 15% and is more common than in adults [3]. Left ventricular hypertrophy is present in 25% to 54% of children [4, 83, 84, 89]. Dilated cardiomyopathy is present in one third [4]. Coronary arteries are involved in 20% to 45% but estimates in children are lacking [87, 93]. Autopsy series have shown coronary artery pathology in 17% including children. Recent series have not documented coronary involvement.
In children, pulmonary arteries are involved in 20.8% and pulmonary hypertension in 15.4% [86]. In an autopsy series of 10 cases, pulmonary artery was involved in two cases with evidence of thromboembolism in one [87]. In a series of 19 children, pulmonary artery stenosis was associated with pulmonary haemorrhage and mortality within 1 month [84].
Valvular involvement is another cardiac manifestation in Takayasu arteritis. Aortic regurgitation (AR) in Takayasu arteritis is primarily due to aortic root dilatation. The prevalence of aortic regurgitation in children is 3 – 24% [83, 84, 94, 95]. A recent study observed moderate to high AR in 46.7% [86]. Mitral regurgitation was present in 13.3% in the same series. In children, valvular involvement can suggest a diagnosis of acute rheumatic fever especially when associated with fever and systemic inflammation, however presence of hypertension and unequal pulses helps in making a diagnosis of Takayasu arteritis [96].
The presence of hypertension in the absence of lower limb pulses can result in an erroneous diagnosis of coarctation of aorta. The presence of pericardial effusion, fever and arthralgias can also result in a mistaken diagnosis of systemic onset juvenile idiopathic arthritis especially in the pre-pulseless phase [97].
The management of juvenile Takayasu arteritis is not different from adults. The exaggerated constitutional symptoms are not consistently reflected by elevation of acute phase reactants. Myocarditis warrants pulse corticosteroids. Cyclophosphamide is more commonly used in children compared to adults in a comparative study [86].
Other paediatric vasculitides
Childhood polyarteritis nodosa (cPAN) may present with cardiac manifestations in 20% [98]. Common presentations include pericarditis, mild valvular regurgitation, cardiac failure and myocardial infarction [98, 99]. Childhood onset eosinophilic granulomatosis with polyangiitis (cEGPA) is another primary vasculitis that has significant cardiac involvement. In a literature review that included 33 children, cardiac involvement was seen in 42%. Cardiomyopathy was present in 47% of those with cardiac involvement and pericardial effusion in 27%. Additionally, severe mitral regurgitation which is not commonly encountered in paediatric rheumatic diseases was noted in 3 children (9%) [100].
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) has a prevalence of 16 to 400 per 100,000 and an incidence of 2 to 20 per 100,000 [101, 102]. JIA is a heterogenous disease with variation in the frequency of different subtypes. Oligoarticular subtype is most common in western population while enthesitis related arthritis and polyarticular JIA are more common in Asian population [101, 103]. Variation in the frequency of subtypes in different geographic areas further complicates estimation of cardiovascular disease.
Pericarditis is the most common cardiac manifestation of JIA and is present in 6.3–36% (Table 2) [104, 105]. It is usually asymptomatic, has mild effusion and mostly resolves on its own with control of disease activity [105]. Symptomatic cardiac involvement is seen in 7.6% and is seen mainly in systemic onset and polyarticular JIA [104].
Among the JIA subtypes, systemic onset JIA (sJIA) is more likely to have pericardial effusion [105]. Recent data suggests pericarditis is present in 7.2% to 28% children with sJIA [106, 107] and cardiac tamponade in 2.5% [108]. There is no difference in cardiac involvement among different subgroups of sJIA, with pericarditis seen in 19% of systemic subtype of sJIA and 15.4% in chronic course of sJIA [108].
Symptomatic myocarditis is seen in 3.5% of children with JIA [104]. More recent series have shown myocarditis to be complicate sJIA in 4.2% [108]. Echocardiographic evidence of subclinical myocarditis is seen in 10% in children with sJIA [104]. A case control study noted children with juvenile arthritis to have lower ejection fractions and larger left ventricular size [109]. Similar echocardiographic observations have been replicated in other cohorts [110, 111]. Symptomatic myocarditis in JIA is associated with a poor outcome [104].
Cardiac abnormalities are detected in 14%—33% when sJIA is complicated by Macrophage activation syndrome (MAS) [112, 119]. Pericardial involvement is seen in 12%—16% [82, 112]. Myocarditis is described in 12% [82]. Pulse corticosteroids is often warranted in this setting. High dose anakinra has been used successfully in fulminant myocarditis complicating MAS in sJIA [120]. Coronary artery dilatation has been described in small series in 16% of sJIA complicated MAS [107]. Coronary artery abnormalities on echocardiography have been described in four children with sJIA who were initially treated as KD but eventually went on to develop arthritis and resolution of coronary echogenicity and dilatation [113]. Pulmonary artery hypertension is also rarely present and has been described in 0.8% [108]. Arrhythmias have also rarely been reported in sJIA MAS [112].
Valvular heart pathologies are described in JIA. Echocardiographic studies have demonstrated mitral valve thickening or regurgitation in 24.3% and aortic valve thickening in 5.4% [111]. Aortic regurgitation has been described in 8 – 10% of HLA B27 associated juvenile spondyloarthropathy(jSpA) [114, 115]. Inflammatory sclerosis of the aortic root is thought to account for its dilatation. This may extend to the valve and to the ventricular septum resulting in conduction abnormalities. However, echocardiographic studies have not consistently shown aortic root dilatation [115]. An early study that included 36 children with jSpA observed echocardiographic evidence of mild mitral regurgitation in 2, and aortic regurgitation in 3 children. Another study on 40 HLA B27 positive JIA children, 4 had aortic valve abnormalities but only one of them had aortic root dilatation. The same study did not find any aortic valve pathology in HLA B27 negative juvenile arthritis controls or healthy children. Mitral valve involvement was also seen but the frequency was not different to controls [114]. More recent cohorts have not shown presence of valvular heart disease [116]. Valvular heart disease has also been described polyarticular JIA. In the pre-DMARD era, Mitral and aortic valvular disease was seen associated with destructive articular disease [104, 121]. Recent data is lacking on valve pathologies specifically in polyarticular JIA.
Premature atherosclerotic cardiovascular disease in juvenile arthritis is increasingly been recognised. A cross sectional study noted a higher carotid intima medial thickness (CIMT) and left ventricular mass index (LVMi) and reduced flow mediated dilatation (FMD) in juvenile idiopathic arthritis between 4 to 18 year [117]. Another study assessing children in the age group 7 to 18 years noted that CIMT was higher in sJIA versus other subtypes [118]. A recent study noted jSpA children had evidence of right ventricular diastolic dysfunction. The authors demonstrated a positive correlation with the juvenile spondyloarthritis disease activity index (jSpADAI). Additionally, those with enthesitis had lower left ventricular function compared to the remaining patients [116].
TNF inhibitors and now JAK inhibitors are part of treatment of JIA. Both are not without safety concerns, especially with regard to cardiovascular complications. However, current evidence from large global trials have allayed cardiac safety concerns of these two drugs in juvenile idiopathic arthritis [122, 123].
Conclusion
Thus, we see that most childhood rheumatic diseases involve the cardiovascular system. Limited long-term follow up data suggests that paediatric rheumatic diseases can lead to ventricular dysfunction and increased risk of atherosclerosis and subsequent cardiovascular morbidity in adulthood. To improve long-term outcomes, early recognition, appropriate and effective treatment with immunosuppressive agents, regular follow up extending into adulthood is needed.
References
Doria A, Iaccarino L, Sarzi-Puttini P et al (2005) Cardiac involvement in systemic lupus erythematosus. Lupus 14:683–686. https://doi.org/10.1191/0961203305lu2200oa
Schwartz T, Diederichsen LP, Lundberg IE et al (2016) Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open 2:e000291. https://doi.org/10.1136/rmdopen-2016-000291
Danda D, Goel R, Joseph G et al (2021) Clinical course of 602 patients with Takayasu’s arteritis: comparison between Childhood-onset versus adult onset disease. Rheumatology (Oxford) 60:2246–2255. https://doi.org/10.1093/rheumatology/keaa569
Misra DP, Aggarwal A, Lawrence A et al (2015) Pediatric-onset Takayasu’s arteritis: clinical features and short-term outcome. Rheumatol Int 35:1701–1706. https://doi.org/10.1007/s00296-015-3272-7
Scalapino K, Arkachaisri T, Lucas M et al (2006) Childhood Onset Systemic Sclerosis: Classification, Clinical and Serologic Features, and Survival in Comparison with Adult Onset Disease. J Rheumatol 33(5):1004–1013
Civieri G, Castaldi B, Martini G et al (2021) Early detection of ventricular dysfunction in juvenile systemic sclerosis by speckle tracking echocardiography. Rheumatology (Oxford) 60:103–107. https://doi.org/10.1093/rheumatology/keaa208
Sozeri B, Deveci M, Dincel N, Mir S (2013) The early cardiovascular changes in pediatric patients with systemic lupus erythematosus. Pediatr Nephrol 28:471–476. https://doi.org/10.1007/s00467-012-2342-2
Barsalou J, Bradley TJ, Silverman ED (2013) Cardiovascular risk in pediatric-onset rheumatological diseases. Arthritis Res Ther 15:212. https://doi.org/10.1186/ar4212
Hersh AO, Trupin L, Yazdany J et al (2010) Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res 62:1152–1159. https://doi.org/10.1002/acr.20179
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Pineles D, Valente A, Warren B et al (2011) Worldwide incidence and prevalence of pediatric onset systemic lupus erythematosus. Lupus 20:1187–1192. https://doi.org/10.1177/0961203311412096
Barron KS, Silverman ED, Gonzales J, Reveille JD (1993) Clinical, serologic, and immunogenetic studies in childhood-onset systemic lupus erythematosus. Arthritis Rheum 36:348–354. https://doi.org/10.1002/art.1780360310
Tucker LB, Menon S, Schaller JG, Isenberg DA (1995) Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Rheumatology (Oxford) 34:866–872. https://doi.org/10.1093/rheumatology/34.9.866
Chang JC, Xiao R, Mercer-Rosa L et al (2018) Child-onset systemic lupus erythematosus is associated with a higher incidence of myopericardial manifestations compared to adult-onset disease. Lupus 27:2146–2154. https://doi.org/10.1177/0961203318804889
Harrison MJ, Zühlke LJ, Lewandowski LB, Scott C (2019) Pediatric systemic lupus erythematosus patients in South Africa have high prevalence and severity of cardiac and vascular manifestations. Pediatr Rheumatol 17:76. https://doi.org/10.1186/s12969-019-0382-x
Guevara JP, Clark BJ, Athreya BH (2001) Point Prevalence of Cardiac Abnormalities in Children with Systemic Lupus Erythematosus. J Rheumatol 28(4):854–859
Gulay CB, Dans LF (2011) Clinical presentations and outcomes of Filipino juvenile systemic lupus erythematosus. Pediatr Rheumatol 9:7. https://doi.org/10.1186/1546-0096-9-7
Oshiro AC, Derbes SJ, Stopa AR, Gedalia A (1997) Anti-Ro/SS-A and anti-La/SS-B antibodies associated with cardiac involvement in childhood systemic lupus erythematosus. Ann Rheum Dis 56:272–274. https://doi.org/10.1136/ard.56.4.272
Chang JC, White BR, Elias MD et al (2019) Echocardiographic Assessment of Diastolic Function in Children with Incident Systemic Lupus Erythematosus. Pediatr Cardiol 40:1017–1025. https://doi.org/10.1007/s00246-019-02107-1
Günal N, Kara N, Akkök N et al (2003) Cardiac abnormalities in children with systemic lupus erythematosus. Turk J Pediatr 45:301–305
Hiraki LT, Benseler SM, Tyrrell PN et al (2008) Clinical and Laboratory Characteristics and Long-Term Outcome of Pediatric Systemic Lupus Erythematosus: A Longitudinal Study. J Pediatr 152:550–556. https://doi.org/10.1016/j.jpeds.2007.09.019
Schanberg LE, Sandborg C, Barnhart HX et al (2009) Premature atherosclerosis in pediatric systemic lupus erythematosus: Risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum 60:1496–1507. https://doi.org/10.1002/art.24469
Cantez S, Gross GJ, MacLusky I, Feldman BM (2017) Cardiac findings in children with juvenile Dermatomyositis at disease presentation. Pediatr Rheumatol 15:54. https://doi.org/10.1186/s12969-017-0182-0
Schwartz T, Sanner H, Husebye T et al (2011) Cardiac dysfunction in juvenile dermatomyositis: a case-control study. Ann Rheum Dis 70:766–771. https://doi.org/10.1136/ard.2010.137968
Witczak BN, Schwartz T, Barth Z et al (2022) Associations between cardiac and pulmonary involvement in patients with juvenile dermatomyositis—a cross-sectional study. Rheumatol Int 42:1213–1220. https://doi.org/10.1007/s00296-021-05071-3
Sampaio-Barros PD, Bortoluzzo AB, Del Rio APT et al (2019) Clinical and laboratory profile of juvenile-onset systemic sclerosis in a Brazilian cohort. J Scleroderma Relat Disord 4:43–48. https://doi.org/10.1177/2397198318769796
Foeldvari I, Tyndall A, Zulian F et al (2012) Juvenile and young adult-onset systemic sclerosis share the same organ involvement in adulthood: data from the EUSTAR database. Rheumatology (Oxford) 51:1832–1837. https://doi.org/10.1093/rheumatology/kes144
Foeldvari I, Klotsche J, Kasapcopur O et al (2021) POS0079 Patients with juvenile systemic sclerosis have a distinct pattern of organ involvement. Results from the juvenile systemic sclerosis inception cohort. Ann Rheum Dis 80(247):2–247. https://doi.org/10.1136/annrheumdis-2021-eular.799
Foeldvari I, Klotsche J, Torok KS et al (2019) Are diffuse and limited juvenile systemic sclerosis different in clinical presentation? Clinical characteristics of a juvenile systemic sclerosis cohort. J Scleroderma Relat Disord 4:49–61. https://doi.org/10.1177/2397198318790494
Borowiec A, Dabrowski R, Wozniak J et al (2012) Cardiovascular assessment of asymptomatic patients with juvenile-onset localized and systemic scleroderma: 10 years prospective observation. Scand J Rheumatol 41:33–38. https://doi.org/10.3109/03009742.2011.609489
Martini G, Foeldvari I, Russo R et al (2006) Systemic sclerosis in childhood: Clinical and immunologic features of 153 patients in an international database. Arthritis Rheum 54:3971–3978. https://doi.org/10.1002/art.22207
Russo RA, Katsicas MM (2007) Clinical characteristics of children with Juvenile Systemic Sclerosis: follow-up of 23 patients in a single tertiary center. Pediatr Rheumatol 5:6. https://doi.org/10.1186/1546-0096-5-6
Rutkowska-Sak L, Gietka P, Gazda A, Kołodziejczyk B (2022) Juvenile systemic sclerosis – observations of one clinical centre. Reumatologia 59(6):367–372. https://doi.org/10.5114/reum.2021.112350
MaríE S-A, Catoggio LJ, Maldonado-Cocco JA et al (1985) Juvenile progressive systemic sclerosis: Clinical and serologic findings. Arthritis Rheum 28:699–702. https://doi.org/10.1002/art.1780280615
Garty BZ, Athreya BH, Wilmott R et al (1991) Pulmonary functions in children with progressive systemic sclerosis. Pediatrics 88:1161–1167
Quartier P, Bonnet D, Fournet J-C et al (2002) Severe cardiac involvement in children with systemic sclerosis and myositis. J Rheumatol 29:1767–1773
Foeldvari I, Zhavania M, Birdi N et al (2000) Favourable outcome in 135 children with juvenile systemic sclerosis: results of a multi-national survey. Rheumatology (Oxford) 39:556–559. https://doi.org/10.1093/rheumatology/39.5.556
Michels H (1997) Course of Mixed Connective Tissue Disease in Children. Ann Med 29:359–364. https://doi.org/10.3109/07853899708999362
Singsen BH, Bernstein BH, Kornreich HK et al (1977) Mixed connective tissue disease in childhood. J Pediatr 90:893–900. https://doi.org/10.1016/S0022-3476(77)80555-6
Mier RJ, Shishov M, Higgins GC et al (2005) Pediatric-Onset Mixed Connective Tissue Disease. Rheum Dis Clin N Am 31:483–496
Rutkowska-Sak L, Gietka P (2019) Clinical features and outcome of mixed connective tissue disease in developmental age – observational study from one center. Reumatologia 57:315–319. https://doi.org/10.5114/reum.2019.91275
Hetlevik SO, Flatø B, Rygg M et al (2017) Long-term outcome in juvenile-onset mixed connective tissue disease: a nationwide Norwegian study. Ann Rheum Dis 76:159–165. https://doi.org/10.1136/annrheumdis-2016-209522
Bidani AK, Roberts JL, Schwartz MM, Lewis EJ (1980) Immunopathology of cardiac lesions in fatal systemic lupus erythematosus. Am J Med 69:849–858. https://doi.org/10.1016/S0002-9343(80)80010-6
Roldan CA, Shively BK, Crawford MH (1996) An Echocardiographic Study of Valvular Heart Disease Associated with Systemic Lupus Erythematosus. N Engl J Med 335:1424–1430. https://doi.org/10.1056/NEJM199611073351903
Leszczynski P, Straburzynska-Migaj E, Korczowska I et al (2003) Cardiac valvular disease in patients with systemic lupus erythematosus Relationship with anticardiolipin antibodies. Clin Rheumatol 22:405–408. https://doi.org/10.1007/s10067-003-0751-0
Gabrielli F, Alcini E, Di Prima MA et al (1995) Cardiac valve involvement in systemic lupus erythematosus and primary antiphospholipid syndrome: lack of correlation with antiphospholipid antibodies. Int J Cardiol 51:117–126. https://doi.org/10.1016/0167-5273(95)02357-3
Jaeggi ET, Hamilton RM, Silverman ED et al (2002) Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. J Am Coll Cardiol 39:130–137. https://doi.org/10.1016/S0735-1097(01)01697-7
Izmirly PM, Costedoat-Chalumeau N, Pisoni CN et al (2012) Maternal Use of Hydroxychloroquine Is Associated With a Reduced Risk of Recurrent Anti-SSA/Ro-Antibody–Associated Cardiac Manifestations of Neonatal Lupus. Circulation 126:76–82. https://doi.org/10.1161/CIRCULATIONAHA.111.089268
Ravelli A, Trail L, Ferrari C et al (2010) Long-term outcome and prognostic factors of juvenile dermatomyositis: A multinational, multicenter study of 490 patients. Arthritis Care Res 62:63–72. https://doi.org/10.1002/acr.20015
Lu Z, Guo-chun W, Li M, Ning Z (2012) Cardiac Involvement in Adult Polymyositis or Dermatomyositis: A Systematic Review. Clin Cardiol 35:685–691. https://doi.org/10.1002/clc.22026
Haupt HM, Hutchins GM (1982) The heart and cardiac conduction system in polymyositis-dermatomyositis: A clinicopathologic study of 16 autopsied patients. Am J Cardiol 50:998–1006. https://doi.org/10.1016/0002-9149(82)90408-8
Denbow CE, Lie JT, Tancredi RG, Bunch TW (1979) Cardiac involvement in polymyositis. Arthritis Rheum 22:1088–1092. https://doi.org/10.1002/art.1780221007
de Diniz M, FR, Kozu KT, Elias AM, et al (2021) Echocardiographic study of juvenile dermatomyositis patients: new insights from speckle-tracking-derived strain. Clin Rheumatol 40:1497–1505. https://doi.org/10.1007/s10067-020-05418-4
Huber AM, Mamyrova G, Lachenbruch PA et al (2014) Early Illness Features Associated With Mortality in the Juvenile Idiopathic Inflammatory Myopathies: Mortality in Juvenile Myositis. Arthritis Care Res 66:732–740. https://doi.org/10.1002/acr.22212
Dedeoglu R, Adroviç A, Oztunç F et al (2017) New Insights into Cardiac Involvement in Juvenile Scleroderma: A Three-Dimensional Echocardiographic Assessment Unveils Subclinical Ventricle Dysfunction. Pediatr Cardiol 38:1686–1695. https://doi.org/10.1007/s00246-017-1714-6
Martini G, Vittadello F, Kasapçopur Ö et al (2009) Factors affecting survival in juvenile systemic sclerosis. Rheumatology (Oxford) 48:119–122. https://doi.org/10.1093/rheumatology/ken388
Ungprasert P, Wannarong T, Panichsillapakit T et al (2014) Cardiac involvement in mixed connective tissue disease: A systematic review. Int J Cardiol 171:326–330. https://doi.org/10.1016/j.ijcard.2013.12.079
Burdt MA, Hoffman RW, Deutscher SL et al (1999) Long-term outcome in mixed connective tissue disease: Longitudinal clinical and serologic findings. Arthritis Rheum 42:899–909. https://doi.org/10.1002/1529-0131(199905)42:5%3c899::AID-ANR8%3e3.0.CO;2-L
Witczak BN, Hetlevik SO, Sanner H et al (2019) Effect on Cardiac Function of Longstanding Juvenile-onset Mixed Connective Tissue Disease: A Controlled Study. J Rheumatol 46:739–747. https://doi.org/10.3899/jrheum.180526
Skagen K, Hetlevik SO, Zamani M et al (2019) Preclinical Carotid Atherosclerosis in Patients With Juvenile-Onset Mixed Connective Tissue Disease. J Stroke Cerebrovasc Dis 28:1295–1301. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.01.027
Crow YJ, Chase DS, Lowenstein Schmidt J et al (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet Part A 167:296–312. https://doi.org/10.1002/ajmg.a.36887
Sönmez HE, Bayındır Y, Batu ED (2023) Cardiovascular manifestations of monogenic periodic fever syndromes. Clin Rheumatol. https://doi.org/10.1007/s10067-023-06504-z
Sener S, Arslanoglu Aydin E, Batu ED (2022) Cardiac involvement and cardiovascular risk factors in pediatric primary systemic vasculitides. Clin Rheumatol 42:673–686. https://doi.org/10.1007/s10067-022-06434-2
Ae R, Makino N, Kosami K et al (2020) Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017–2018. J Pediatr 225(23–29):E2. https://doi.org/10.1016/j.jpeds.2020.05.034
Kato K, Sullivan PF, Evengård B, Pedersen NL (2006) Chronic Widespread Pain and Its Comorbidities: A Population-Based Study. Arch Intern Med 166:1649–1654. https://doi.org/10.1001/archinte.166.15.1649
Terai M, Shulman ST (1997) Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr 131:888–893. https://doi.org/10.1016/S0022-3476(97)70038-6
Newburger JW, Takahashi M, Burns JC et al (1986) The Treatment of Kawasaki Syndrome with Intravenous Gamma Globulin. N Engl J Med 315:341–347. https://doi.org/10.1056/NEJM198608073150601
Furusho K, Nakano H, Shinomiya K et al (1984) HIGH-dose intravenous gammaglobulin for kawasaki disease. The Lancet 324:1055–1058. https://doi.org/10.1016/S0140-6736(84)91504-6
Uehara R, Belay ED (2012) Epidemiology of Kawasaki Disease in Asia, Europe, and the United States. J Epidemiol 22:79–85. https://doi.org/10.2188/jea.JE20110131
Akagi T, Kato H, Inoue O et al (1990) Valvular heart disease in Kawasaki syndrome: Incidence and natural history. Am Heart J 120:366–372. https://doi.org/10.1016/0002-8703(90)90081-8
Yutani C, Go S, Kamiya T et al (1981) Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med 105:470–473
Printz BF, Sleeper LA, Newburger JW et al (2011) Noncoronary Cardiac Abnormalities Are Associated With Coronary Artery Dilation and With Laboratory Inflammatory Markers in Acute Kawasaki Disease. J Am Coll Cardiol 57:86–92. https://doi.org/10.1016/j.jacc.2010.08.619
Moran AM, Newburger JW, Sanders SP et al (2000) Abnormal myocardial mechanics in Kawasaki disease: Rapid response to gamma-globulin. Am Heart J 139:217–223
Suzuki A, Kamiya T, Tsuchiya K et al (1988) Tricuspid and mitral regurgitation detected by color flow Doppler in the acute phase of Kawasaki disease. Am J Cardiol 61:386–390. https://doi.org/10.1016/0002-9149(88)90950-2
Ravekes WJ, Colan SD, Gauvreau K et al (2001) Aortic root dilation in kawasaki disease. Am J Cardiol 87:919–922. https://doi.org/10.1016/S0002-9149(00)01541-1
Kanegaye JT, Wilder MS, Molkara D et al (2009) Recognition of a Kawasaki Disease Shock Syndrome. Pediatrics 123:e783–e789. https://doi.org/10.1542/peds.2008-1871
Oates-Whitehead RM, Baumer JH, Haines L, et al (2003) Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev 2021. https://doi.org/10.1002/14651858.CD004000
McCrindle BW, Rowley AH, Newburger JW et al (2017) Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 135:e928–e999
Wallace CA, French JW, Kahn SJ, Sherry DD (2000) Initial Intravenous Gammaglobulin Treatment Failure in Kawasaki Disease. Pediatrics 105:e78–e78. https://doi.org/10.1542/peds.105.6.e78
Riphagen S, Gomez X, Gonzalez-Martinez C et al (2020) Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet 395:1607–1608. https://doi.org/10.1016/S0140-6736(20)31094-1
Sperotto F, Friedman KG, Son MBF et al (2021) Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 180:307–322. https://doi.org/10.1007/s00431-020-03766-6
Otar Yener G, Paç Kısaarslan A, Ulu K et al (2022) Differences and similarities of multisystem inflammatory syndrome in children, Kawasaki disease and macrophage activating syndrome due to systemic juvenile idiopathic arthritis: a comparative study. Rheumatol Int 42:879–889. https://doi.org/10.1007/s00296-021-04980-7
Goel R, Kumar TS, Danda D et al (2014) Childhood-onset Takayasu Arteritis — Experience from a Tertiary Care Center in South India. J Rheumatol 41:1183–1189. https://doi.org/10.3899/jrheum.131117
Cakar N, Yalcinkaya F, Duzova A et al (2008) Takayasu Arteritis in Children. J Rheumatol 35:913–919
Kerr GS, Hallahan CW, Giordano J et al (1994) Takayasu Arteritis. Ann Intern Med 120:919–929. https://doi.org/10.7326/0003-4819-120-11-199406010-00004
Bolek EC, Kaya Akca U, Sari A et al (2021) Is Takayasu’s arteritis more severe in children? Clin Exp Rheumatol 39:32–38. https://doi.org/10.55563/clinexprheumatol/kr357t
Sharma BK, Jain S, Radotra BD (1998) An autopsy study of Takayasu arteritis in India. Int J Cardiol 66:S85–S90. https://doi.org/10.1016/S0167-5273(98)00155-7
Lupi-Herrera E, Sánchez-Torres G, Marcushamer J et al (1977) Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J 93:94–103. https://doi.org/10.1016/S0002-8703(77)80178-6
Jain S, Sharma N, Singh S et al (2000) Takayasu Arteritis in children and young Indians. Int J Cardiol 75:S153–S157. https://doi.org/10.1016/S0167-5273(00)00180-7
Hahn D, Thomson PD, Kala U et al (1998) A review of Takayasu’s arteritis in children in Gauteng, South Africa. Pediatr Nephrol 12:668–675. https://doi.org/10.1007/s004670050526
Talwar KK, Chopra P, Narula J et al (1988) Myocardial involvement and its response to immunosuppressive therapy in nonspecific aortoarteritis (Takayasu’s disease) — a study by endomyocardial biopsy. Int J Cardiol 21:323–334. https://doi.org/10.1016/0167-5273(88)90109-X
Talwar KK, Kumar K, Chopra P et al (1991) Cardiac involvement in nonspecific aortoarteritis (Takayasu’s arteritis). Am Heart J 122:1666–1670. https://doi.org/10.1016/0002-8703(91)90285-P
Hotchi M (1992) Pathological studies on Takayasu arteritis. Heart Vessels 7:11–17. https://doi.org/10.1007/BF01744538
Eleftheriou D, Varnier G, Dolezalova P et al (2015) Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther 17:36
Jales-Neto L, Levy-Neto M, Bonfa E et al (2010) Juvenile-onset Takayasu arteritis: peculiar vascular involvement and more refractory disease. Scand J Rheumatol 39:506–510. https://doi.org/10.3109/03009741003742730
Ravelli A, Pedroni E, Perrone S et al (1999) Aortic valve regurgitation as the presenting sign of Takayasu arteritis. Eur J Pediatr 158:281–283. https://doi.org/10.1007/s004310051072
Harahsheh AS, Kulkarni A, Becker C, Ross RD (2007) Conditions Mimicking Coarctation of the Aorta. Pediatr Cardiol 28:385–388. https://doi.org/10.1007/s00246-007-0038-3
Ozen S, Besbas N, Saatci U, Bakkaloglu A (1992) Diagnostic criteria for polyarteritis nodosa in childhood. J Pediatr 120:206–209. https://doi.org/10.1016/S0022-3476(05)80428-7
Günal N, Kara N, Çakar N et al (1997) Cardiac involvement in childhood polyarteritis nodosa. Int J Cardiol 60:257–262. https://doi.org/10.1016/S0167-5273(97)00119-8
Zwerina J, Eger G, Englbrecht M et al (2009) Churg-Strauss Syndrome in Childhood: A Systematic Literature Review and Clinical Comparison with Adult Patients. Semin Arthritis Rheum 39:108–115. https://doi.org/10.1016/j.semarthrit.2008.05.004
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. The Lancet 369:767–778. https://doi.org/10.1016/S0140-6736(07)60363-8
Koca B, Sahin S, Adrovic A et al (2017) Cardiac involvement in juvenile idiopathic arthritis. Rheumatol Int 37:137–142. https://doi.org/10.1007/s00296-016-3534-z
Naveen R, Jain A, Muhammed H et al (2021) Macrophage activation syndrome in systemic lupus erythematosus and systemic-onset juvenile idiopathic arthritis: a retrospective study of similarities and dissimilarities. Rheumatol Int 41:625–631. https://doi.org/10.1007/s00296-020-04763-6
Goldenberg J, Ferraz MB, Pessoa AP et al (1992) Symptomatic cardiac involvement in juvenile rheumatoid arthritis. Int J Cardiol 34:57–62. https://doi.org/10.1016/0167-5273(92)90082-E
Bernstein B, Takahashi M, Hanson V (1974) Cardiac involvement in juvenile rheumatoid arthritis. J Pediatr 85:313–317. https://doi.org/10.1016/S0022-3476(74)80107-1
Ruscitti P, Natoli V, Consolaro A et al (2022) Disparities in the prevalence of clinical features between systemic juvenile idiopathic arthritis and adult-onset Still’s disease. Rheumatology (Oxford) 61:4124–4129. https://doi.org/10.1093/rheumatology/keac027
Felix A, Delion F, Suzon B et al (2022) Systemic juvenile idiopathic arthritis in French Afro-Caribbean children, a retrospective cohort study. Pediatr Rheumatol 20:98. https://doi.org/10.1186/s12969-022-00766-8
Neau P-A, El-Jammal T, Javaux C et al (2022) The Spectrum of Still’s Disease: A Comparative Analysis of Phenotypic Forms in a Cohort of 238 Patients. J Clin Med 11:6703. https://doi.org/10.3390/jcm11226703
Bharti BB, Kumar S, Kapoor A et al (2004) Assessment of left ventricular systolic and diastolic function in juvenile rheumatoid arthritis. J Postgrad Med 50:262–265 (discussion 266-267)
Oguz D, Ocal B, Ertan Ü et al (2000) Left Ventricular Diastolic Functions in Juvenile Rheumatoid Arthritis. Pediatr Cardiol 21:374–377. https://doi.org/10.1007/s002460010084
Alkady EAM, Helmy HAR, Mohamed-Hussein AAR (2012) Assessment of cardiac and pulmonary function in children with juvenile idiopathic arthritis. Rheumatol Int 32:39–46. https://doi.org/10.1007/s00296-010-1548-5
Minoia F, Davì S, Horne A et al (2014) Clinical Features, Treatment, and Outcome of Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A Multinational, Multicenter Study of 362 Patients: Macrophage Activation Syndrome in Systemic JIA. Arthritis Rheumatol 66:3160–3169. https://doi.org/10.1002/art.38802
Lefèvre-Utile A, Galeotti C, Koné-Paut I (2014) Coronary artery abnormalities in children with systemic-onset juvenile idiopathic arthritis. Joint Bone Spine 81:257–259. https://doi.org/10.1016/j.jbspin.2013.09.004
Huppertz H-I, Voigt I, Müller-Scholden J, Sandhage K (2000) Cardiac Manifestations in Patients with HLA B27-Associated Juvenile Arthritis. Pediatr Cardiol 21:141–147. https://doi.org/10.1007/s002469910023
Stamato T, Laxer RM, de Freitas C et al (1995) Prevalence of cardiac manifestations of juvenile ankylosing spondylitis. Am J Cardiol 75:744–746. https://doi.org/10.1016/S0002-9149(99)80672-9
Yildiz M, Dedeoglu R, Akdeniz B et al (2022) Systolic and Diastolic Cardiac Functions in Juvenile Spondyloarthropathies. JCR J Clin Rheumatol 28:e175–e179. https://doi.org/10.1097/RHU.0000000000001674
Hussain KS, Gulati R, Satheesh S, Negi VS (2021) Early-onset subclinical cardiovascular damage assessed by non-invasive methods in children with Juvenile Idiopathic Arthritis: analytical cross-sectional study. Rheumatol Int 41:423–429. https://doi.org/10.1007/s00296-020-04689-z
Vlahos AP, Theocharis P, Bechlioulis A et al (2011) Changes in vascular function and structure in juvenile idiopathic arthritis. Arthritis Care Res 63:1736–1744. https://doi.org/10.1002/acr.20613
Çakan M, Karadağ ŞG, Tanatar A, Ayaz NA (2020) The frequency of macrophage activation syndrome and disease course in systemic juvenile idiopathic arthritis. Mod Rheumatol 30:900–904. https://doi.org/10.1080/14397595.2019.1660026
Meneghel A, Martini G, Amigoni A et al (2021) Case Report: Life-Threatening Macrophage Activation Syndrome With Fulminant Myocarditis Successfully Rescued by High Dose Intravenous Anakinra. Front Pediatr 8:635080. https://doi.org/10.3389/fped.2020.635080
Özer S, Alehan D, Özme S et al (1994) Mitral and aortic insufficiency in polyarticular juvenile rheumatoid arthritis. Pediatr Cardiol 15:151–153. https://doi.org/10.1007/BF00796329
Burmester GR, Panaccione R, Gordon KB et al (2013) Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 72:517–524. https://doi.org/10.1136/annrheumdis-2011-201244
Ruperto N, Brunner HI, Synoverska O et al (2021) Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. The Lancet 398:1984–1996. https://doi.org/10.1016/S0140-6736(21)01255-1
Author information
Authors and Affiliations
Contributions
KT: Wrote the draft, revised it and gave approval for the final version.
AA: Conception, critical revision of the manuscript, approval of the final manuscript.
All co-authors take full responsibility for the integrity and accuracy of the work.
Corresponding author
Ethics declarations
Disclosures
None
Disclaimer
No text or graphs have been copied.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript has not been submitted or published anywhere.
This article is part of the Topical Collection on Cardiovascular Issues in Rheumatic Diseases
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, K.N., Aggarwal, A. Childhood rheumatic diseases: bites not only the joint, but also the heart. Clin Rheumatol 42, 2703–2715 (2023). https://doi.org/10.1007/s10067-023-06621-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06621-9