Abstract
Introduction
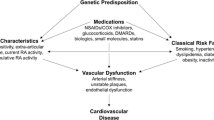
Rheumatoid arthritis (RA) is a systemic autoimmune disease with important cardiovascular (CV) implications. CV disease represents over half of RA patient deaths and causes significant morbidity. CV manifestations in RA can be complex, raising concerns for adequate patient management and provider-dependent roles.
Methods
This is a retrospective study of patients diagnosed with RA and coronary artery disease (CAD). Patients were identified and filtered via EPIC Database search engine. Parameters were set from January 1, 2014, to December 31, 2020. Inclusion criteria consisted of patients who met diagnostic criteria for both RA and CAD. A total of 399 patients met criteria.
Results
Of the 399 identified patients, 272 were female (68.2%) and 127 were male (31.8%) with a median age of 73 (range 26–98). The population was further divided into two groups: those with established cardiology care versus those without. Patients without cardiology follow-up experienced significantly more hospitalizations (RR 1.63 95% CI 1.12, 2.38), higher rates of adverse events including myocardial infarction (MI) (RR 4.82 95% CI 1.94, 11.98), heart failure (HF) (OR 15.81 95% CI 3.54, 70.52), and stroke (RR 2.55 95% CI 1.29, 5.03). Patients not followed by cardiology also had numerical increases in CV death (4 deaths compared to none in those with cardiology follow) and all-cause mortality (HR 1.03 95% CI 0.63, 1.67).
Conclusion
Patients with regular cardiology follow-up demonstrated fewer cardiac-related adverse events. This suggests that co-management may have a role in adverse cardiac event risk reduction and should therefore be an early consideration.
Key Points |
• Rheumatoid arthritis patients demonstrate higher rates of coronary disease compared to the general population. Traditional cardiac risk factors may not be entirely responsible for this phenomenon • Hospitalization rates and adverse event occurrence are significantly higher in patients with single-provider care (rheumatology only) compared to dual provider care (rheumatology and cardiology) • Cardiology co-management should be an early consideration in the management of RA patients • Early screening, risk stratification of coronary disease, and utilization of appropriate treatment algorithms are important to decrease morbidity and mortality |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune condition that affects approximately 0.5–1.0% of the population [1, 2]. This chronic inflammatory polyarthritis results in erythema, edema, and gradual destruction of synovial joints [1–4]. RA patients can also experience extra-articular manifestations and have a reduced life expectancy compared to the general population [3, 5–9].
Cardiovascular (CV) death is a leading cause of mortality in patients with RA, with several studies suggesting that it is solely responsible for over half of deaths observed in RA cohorts [3, 5–7]. Despite recent advancements, it is unclear why rheumatoid patients have higher rates of coronary disease when compared to the general population [3]. Collective literature findings suggest that traditional cardiovascular risk factors are not entirely responsible for the increased CV risk. A growing amount of evidence proposes that inflammation plays a key role in the pathogenesis of atherosclerotic plaque formation, which leads to increased morbidity from CV disease (CVD) [2, 7, 9–14].
The aim of the study is to compare patient outcomes in those patients seen by rheumatology alone vs patients co-managed by cardiology and rheumatology in a real-world clinical setting. Advances in the comprehension of trends, interventions, and management of such complex patients not only serve to improve understanding of the pathogenesis of CAD in RA, but also to optimize treatment of such patients.
Study design and methods
Upon receiving Institutional Review Board (IRB) approval (BSW #357,760), we retrospectively identified 1249 patients with the utilization of EPIC Database specialized search engine through input of International Classification of Diseases (ICD-10) codes for RA and CAD, including subtypes and variations (Supplement 1). Parameters were set from January 1, 2014, to December 31, 2020. Inclusion criteria consisted of patients who met diagnostic criteria for RA and CAD, 18 years or older, and those exclusively followed by College Station, Round Rock, and Temple TX BSW Health Rheumatologists. Exclusion criteria included patients not followed by a Rheumatologist in any of these regions, those without a formal diagnosis of RA or CAD, or any duplicate entries.
A total of 399 out of 1249 patient charts met criteria. To facilitate statistical analyses, the patient cohort was split into two groups: group I: followed by Cardiology and Rheumatology “Cardio-Rheum” and group II: followed by rheumatology only “Rheum.” Each group was retrospectively analyzed for the number of hospitalizations and adverse event occurrence within the 6-year period. Furthermore, the hospitalizations themselves were classified into either CV or non-CV causes. CV hospitalizations consisted of any hospitalization related to cardiac causes, to include angina, non-ST-segment elevation myocardial infarction (NSTEMI), ST-segment elevation myocardial infarction (STEMI), heart failure (HF), arrhythmia of any type, valvular heart disease, pericardial effusion, and other cardiac emergencies such as tamponade, aortic dissection, or hospitalizations related to peripheral vascular disease, limb ischemia, or stroke. Planned surgical interventions, to include scheduled revascularization procedures such as percutaneous coronary intervention (PCI), were not counted toward the hospitalization rate nor were they counted as adverse events. These interventions were considered planned outpatient interventions; myocardial infarctions (MIs) occurring without warning in an uncontrolled setting were considered adverse events.
Primary and secondary outcomes
Hospitalizations and adverse events represented co-primary outcomes in this study. Secondary outcomes focused on the components of the primary outcome via breakdown of cohort data into CV versus non-CV hospitalizations. Further data points included the measure of healthcare utilization metrics consisting of number of cardiology clinic visits, established outpatient cardiology follow-up, time to first cardiology visit after RA diagnosis, number of events prior to first cardiology visit, and length of RA disease in relation to CV diagnosis, treatment, and outcome.
Classification of coronary artery disease and adverse events
In reference to numerous studies [2, 15–17], we define the spectrum of CAD as:
-
1.Prior coronary revascularization with PCI or coronary artery bypass graft surgery (CABG).
-
2.History of hospitalization related to acute coronary syndrome (ACS), including NSTEMI or STEMI.
-
3.History of abnormal stress test(s).
-
4.Ischemia with non-obstructive CAD (INOCA) defined as a coronary artery stenosis 20% or greater but less than 70% in an epicardial coronary artery or less than 50% in the left main coronary artery, as recorded in coronary angiography reports.
-
a. This can also include any angiographic recording of significance with a negative physiologic study (fractional flow reserve or non-hyperemic resting pressure indices).
-
5. Normal coronary arteries.
Hospitalized MIs were defined according to American Academy of Cardiology (ACC) Guidelines [18]. Numerous published guidelines were utilized in the classification of the other listed adverse events [18–21]. Adverse events included: STEMI, NSTEMI, angina, hospitalization for new-onset HF, stroke, CV death, and all-cause mortality.
We defined angina as exertional versus non-exertional or transient ECG changes suggestive of acute ischemia without troponin elevation. New HF diagnosis consisted of patients presenting with first-time evidence of volume overload explained by either heart failure with preserved ejection fraction (HFpEF) or heart failure with reduced ejection fraction (HFrEF) attributed to several conditions including hypertensive heart disease, arrhythmia-induced, valvular-related, as a direct consequence of MI, drug-induced, viral, idiopathic, or amyloid-based cardiomyopathies. Stroke included CV and non-CV causes. Cardiac etiologies of stroke included thromboembolic sources due to arrhythmia, valvular pathologies, or structural heart abnormalities such as patent foramen ovale (PFO).
Cardiovascular death was defined as cardiac arrest as a direct result of MI and “all-cause mortality” looked at any other cause of death. Both were logged as hospitalization occurrences from the time of admission, up to 72 h post-discharge. A separate mortality count, “all-time mortality” was recorded to account for those deaths outside of this range but included within the 6-year timeframe.
Statistical methods
Descriptive statistics summarizing the characteristics of the study population were calculated for demographic, behavioral, anthropometric, medical comorbid, and endothelial function measures. Frequencies and percentages were used to describe categorical variables. Means and standard deviations were used to describe continuous variables. The chi-square test (Fisher exact test when low counts were present) tested for associations in bivariate comparisons. The Cochran-Armitage Trend test was used to identify trends in rates over ordinal variables while two-sample t-tests (Wilcoxon rank-sum test when appropriate) were utilized to test for differences in continuous variables between 2 groups. Wilcoxon signed-rank tests were used to assess changes in hospitalization utilization before and after cardiology involvement. All tests were two-tailed with a (0.05) type I error rate, except for those examining the individual components of the primary outcome [22, 23]. In order of most to least important, the hierarchy is as follows: MI, cardiovascular death, all-cause mortality, cardiovascular hospitalization, and stroke.
Rates of adverse events were calculated as event rates per year to account for differing observation times. Differences in event rates between the Cardio-Rheum and Rheum group were analyzed using Wilcoxon rank-sum tests to account for the skewed nature of the data. For the intervention group Cardio-Rheum, observation time was defined as time from CV intervention or starting on January 1, 2014, whichever came later, to time of death, or December 31, 2020, whichever came first. Observation time for the control group Rheum was defined as time from RA diagnosis, or January 1, 2014, until December 31, 2020, or death.
Results
Cohort characteristics
Patient demographics included a total of 399 patients: 272 women (68.2%) and 127 men (31.8%) with a median age of 73 (IQR, − 66–80). Cohort demographic data, including a breakdown of Cardio-Rheum and Rheum, is summarized in Table 1 and Table 2.
Cardio-Rheum cohort
Most patients in Cardio-Rheum had at least 1 adverse event prior to establishing care (77.8%). Further data breakdown demonstrated 40.0% of patients had just one adverse event, followed by 20.4% with two, and 17.4% with three or more adverse events prior to establishing care. Once patients established care with cardiology, they averaged 8.35 clinic visits (range, 1–35) throughout the period in which they were followed. Following the implementation of co-management, 74.5% of patients in the Cardio-Rheum group did not have any further CV-related hospitalizations. Of those that were hospitalized after establishing care (25.5%), the mean number of CV hospitalizations was 1.49 visits (range, 1–5) with no patient hospitalized more than 5 times. A significant decrease (mean, − 1.1 visit difference, p < 0.0001) in CV-related hospitalizations after establishing care was determined (p < 0.0001) (Fig. 1).
In terms of non-CV-related hospitalizations, only 23.2% of patients did not have any further hospitalizations, while the majority did have at least one (76.8%). The average number of non-CV hospitalizations while under Cardiology and Rheumatology co-management was 4 (range 0–36). About 19.0% of patients had greater than 5 non-CV hospitalizations. The difference between CV and non-CV hospitalizations was determined to be significant (p < 0.0001). Although patients with established cardiology and rheumatology care did have a significant decrease in the number of CV-related hospitalizations, there was no significant change or decrease in all other hospitalization types. Hospital utilization in the Cardio-Rheum cohort is summarized in Table 3.
Finally, CV and non-CV hospitalizations were evaluated based on duration of RA disease. A Cochran-Armitage test was performed to assess if the proportion of CV hospitalizations increased or decreased over the length of RA disease; a significant difference was not detected (p = 0.1986). The same held true for non-CV hospitalizations (p = 0.4030).
Between-group analyses: Cardio-Rheum compared to Rheum
Hospitalized MIs were > 2.71 times more prevalent in Rheum compared to Cardio-Rheum patients (p < 0.0001). In fact, those not followed by cardiology experienced significantly higher rates of all cardiac adverse events including angina, NSTEMI, STEMI (RR 4.82 95% CI 1.94, 11.98) p < 0.001, and new-onset HF (OR 15.81 95% CI 3.54, 70.52). The occurrence of stroke in Rheum was 22 compared to 15 total events in Cardio-Rheum (p < 0.0048).
Adverse event occurrence in both cohorts is summarized in Table 4. Approximately (12.9%) of Cardio-Rheum patients had at least one adverse event compared to (50.0%) of the Rheum cohort. The most common adverse event was stroke (5.54%), followed by NSTEMI (2.21%). There were 11 all-cause mortalities—but no cardiovascular deaths in the 6-year window.
The mean length of follow-up in Cardio-Rheum was 50.1 (± 25.6) months compared to Rheum who demonstrated a mean follow-up of 58.6 months (± 26.1; p = 0.0003).
Mortality
We identified no CV deaths in Cardio-Rheum compared to 4 CV deaths in Rheum. Gray’s test (an equivalent to the log-rank test that accounts for the competing risk of death outside of hospital admission) was used to assess for a significant difference in cumulative incidence of death between the two groups with a significant difference detected (p = 0.0178). We identified 11 and 16 non-cardiac deaths in Cardio-Rheum and Rheum cohorts, respectively (p = 0.0004). All-time mortality on the other hand did not demonstrate a significant difference via log-rank test (p = 0.9199) (Fig. 2a–c).
Discussion
In this retrospective cohort study, patients without established cardiology care had significantly more CV hospitalizations, adverse events, and an overall increased rate of in-hospital mortality from both cardiac and non-cardiac causes. This suggests that patients with RA who are co-managed by cardiology and rheumatology are less likely to end up hospitalized after care is established with both services. Even when Cardio-Rheum patients ended up hospitalized, they experienced significantly less adverse events compared to Rheum.
This trend was not observed on “all-time mortality” which could be explained by several reasons. The most likely explanation for this can be attributed to death due to natural phenomena. Our patient population was, on average, 72.3 years of age and the average lifespan for a citizen of the USA is 77.0 years [24]. It is also important to note that a sizable portion (almost one-third) of the population had no cardiology follow-up. These patients were either followed by primary care physicians in internal medicine or family medicine and rheumatology, or managed by rheumatology alone. While care may not have been optimized to effectively reduce in-hospital adverse events and mortality, they still received routine, high-quality medical care with adequate follow-up. Patients without cardiology co-management had significantly longer follow-up times, which could speak to lack of efficiency (compared to the co-managed cohort) attributed to specialized medical care, but also supports the notion of a patient population with dedicated, long-term care. Finally, a cohort of roughly 400 patients may not have been powered to detect a significant difference in all-time mortality.
Other considerations
This patient population does not represent a low-risk cohort. In fact, most Cardio-Rheum patients (77.8%) had at least one CV event prior to establishing care with cardiology. This can possibly be attributed to patient reluctance to initiate care, lack of patient referral post-hospitalization, and personal reasons relating to access, missed appointments, or due to financial reasons [25]. Another factor to consider is the impact of the COVID-19 pandemic, which has been shown to have contributed to patient reluctance to seek care [26]. This is also a testament to the notion that early detection of CAD proves to be one of the most daunting challenges in terms of cardiac management of RA patients [3]. More importantly, it speaks to the notion of “Silent MI”—a trend that has been previously studied and identified in the RA patient population [3, 27–30].
Maradit-Kramers et al. discussed the controversies surrounding the magnitude of coronary heart disease (CHD) risk, clinical presentation, and outcomes in RA patients. The study determined that RA patients had a significantly higher risk of both hospitalized and unrecognized MI prior to the incidence of RA—suggesting that the development of CHD in the RA patient precedes the diagnosis of RA. The study also suggested that patients with RA are less likely to demonstrate symptoms of angina, less likely to undergo CABG, and demonstrate a significantly higher chance of sudden death compared to the non-RA cohort [3].
Sheifer et al. described a review of unrecognized MI as a clinical conundrum, often mistaken for other diseases such as end-stage renal disease or manifestations of diabetes mellitus (DM) [27]. Unrecognized MIs are believed to constitute up to 30% of MIs detected. The discrepancy can be attributed to several factors including difficulty in ascertaining pain by individual perception, (including the poor dermatomal distribution of pain associated with MI) and remarkably enough, cytokine balance [28–30]. There is a theory that among RA patients, although the experience of angina itself may be equally prevalent in both RA and non-RA patients, RA patients may be less inclined to seek medical attention for this symptom and are much more likely to attribute it to that of chronic inflammation, such as arthritis [3, 27, 28]. These considerations are magnified in this study’s cohort, where—despite a systematic review of RA patients—the majority presented with at least one adverse cardiac event prior to establishing care with cardiology. These findings suggest that there may be deficits in effectively screening, detecting, and treating CAD in a timely manner.
Our selection and screening criteria relied on pre-established CAD—regardless of expression of symptoms. The possibility of having a patient population with heavier reliance of CAD diagnosis based on symptomatic presentation cannot be excluded, however. In the end, this population was not a focus in this study. Despite this, it is worth noting that our study population did not demonstrate significant change in terms of number of hospitalizations over the length of RA disease.
Cohort comorbidities
Classically, RA cohorts have demonstrated undiagnosed, poorly managed comorbidities in various studies [31]. In our patient population, it is important to note that most patients had established hypertension (HTN) and hyperlipidemia (HLD). It is important to note that unlike other cohorts from similar, previously studied populations, these patients had accurately diagnosed comorbidities, as this was the way they were identified via electronic medical record search. Previous studies have cited most RA patient populations as underdiagnosed and undertreated for their present comorbidities, to include HTN, HLD, and DM [31].
Cardiovascular risk prediction algorithms in rheumatic diseases
Clinical prediction algorithms presently used in risk stratifying rheumatic patients for cardiovascular risk are inconsistent and often misclassify RA patients’ CV risk [32, 33]. A recently published review found that general risk algorithms mostly underestimate and occasionally overestimate cardiovascular risk in rheumatic patients [34]. Colaco et al. discussed CV risk prediction algorithms focused on studies involving subjects with RA, ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), or psoriasis, who utilized at least one CV risk prediction algorithm. The review highlighted important findings. When RA patients are CV risk stratified, the Framingham Risk Score (FRS) is the most frequently used calculator, but the observed risk is 1.8-times higher than the predicted risk. This is especially true in women, seropositive RA, and those with persistently elevated inflammatory markers. Even in instances where Reynold’s Risk Score (RRS) calculator was used to account for CRP, there were similar deficits in observed vs predicted risk [33]. Application of the 2016 European League Against Rheumatism (EULAR) 1.5-times multiplier to the FRS and Pooled Cohort Equations (PCE), while helpful, overestimated future CV risk and thus, failed to discriminate effectively [34, 35]. Despite multiple attempts at rectifying and re-establishing an accurate CV risk score (to include the addition of autoantibodies and inflammatory biomarkers such as CRP) to the FRS, QRISK2 proved fruitless. Lastly, newly designed models such as The Expanded Risk Score in Rheumatoid Arthritis (ERS-RA) calculator have been developed in an attempt to establish an accurate RA CV risk protocol. It was also found to overestimate CV risk and presently demonstrates an overall inferior discriminatory function compared to its predecessors.
No universally accepted risk algorithm or calculator has been developed. Risk algorithms generally underestimate and, at times, overestimate CV risk in rheumatoid arthritis and psoriatic arthritis patients. Such patients’ CV risk cannot be easily identified with traditional CV risk factors alone. Until a new risk calculator is developed and validated, RA patients will require a more involved risk classification. Such findings highlight the importance of close follow-up and monitoring to improve stratification, with co-management serving as a vital option early in disease course. As evident in this study, patients who had regular cardiology follow-up fared much better in terms of reduction of adverse cardiovascular outcomes. Despite the existence of standardized risk stratification, patients should be referred to the cardiology service more regularly and promptly.
Improving the screening and management of cardiovascular risk factors and disease in the rheumatology setting: the establishment of a cardiology-rheumatology clinic
EULAR developed recommendations for cardiovascular disease risk prediction and management focused on RA and other inflammatory joint disorders that can be applied to our studied population. The three main principles discussed by the multidisciplinary task force include: 1. being aware of the higher risk for CVD in patients with RA compared to the general population, 2. ensuring CVD risk stratification is performed, and 3. NSAID and corticosteroid utilization should be in accordance with treatment specific recommendations [35].
There have been several strategies suggested in the literature for outlining provider roles in a multidisciplinary approach to implement available CVD risk reduction recommendations. These include tracking of traditional risk factors, medications, and communication methods between Cardiology, Rheumatology, and Primary Care providers involved in the care of RA patients [36–38]. Communication deficiencies between providers as well as low diabetes and lipid screening rates have been previously identified as a barrier to the implementation of guideline recommendations [31].
Limitations and future directions
Identification of RA-specific medications was not a focus of this study; however, it is important to understand the impact of their role as related to CVD risk. Glucocorticoids can significantly increase cardiovascular risk, particularly with prolonged courses and higher dose administration. Current EULAR recommendations include establishing clear plans for the tapering and discontinuation of steroids as soon as clinically feasible. Disease-modifying antirheumatic drugs (DMARDs) including TNF-alpha inhibitors have also been drugs of interest given several studies pointing towards a cardioprotective effect associated to their use in RA patients, primarily thought to be related to their anti-inflammatory properties and prevention of atherosclerosis progression. Further investigations are warranted to elucidate any significant associations between RA-specific medication use and cardiovascular outcomes. In this study, data was identified and organized via documented office visits and hospitalizations as opposed to specific medication regimens. Our population demonstrated a degree of heterogeneity in this regard, which could not be statistically accounted for.
The occurrence of adverse events was a primary point of interest. An attempt was made to analyze adverse event occurrences between CV and non-CV hospitalizations. The data proved to be too sparse for statistical analyses given the absence of adverse event subtypes. Adverse event occurrences were therefore combined for the two hospitalization types. Finally, the results of this study represent a Central Texas population, of which the majority was Caucasian. The findings may not be representative of some other minorities within the USA or regions of the world with unique environmental and genetic predispositions.
Methods to facilitate the implementation of expert advice and evidence-based approaches to optimize the care of RA patients and improve cardiovascular outcomes are still needed. Further research focus can be geared towards the implementation of an integrated cardiology-rheumatology clinic as a future prospective pilot study in co-management, with potential future data providing exciting possibilities to impact clinical practice.
Conclusions
Given the increased risk of morbidity and mortality related to CV disease and the silent nature of CAD, intervention via cardiology co-management should be an early consideration in the management of RA patients. Further studies are warranted to identify additional interventions for CV disease risk reduction in this select patient population. The findings of this study may potentially provide a blueprint to guide medical management and optimize outcomes in patients with both medical conditions. Early screening, risk stratification of coronary disease, and appropriate treatment algorithms are important to decrease morbidity and mortality.
References
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JMW, Hobbs K, Huizinga TWJ, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky ́ J, Wolfe F, Hawker G (2010) Rheumatoid Arthritis Classification Criteria. An American College of Rheumatology/ European League Against Rheumatism Collaborative Initiative. Arthritis Rheum 62(9):2569–2581
Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, Pincus T, Avalos I, Stein CM (2005) Increased coronary-artery atherosclerosis in rheumatoid arthritis. Arthritis Rheum 52(10):3045–3053
Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis A population-based cohort study. Arthritis Rheum 52(2):402–411. https://doi.org/10.1002/art.20853
Li J (2004) Silent myocardial ischemia may be related to inflammatory response. Med Hypotheses 62:252–256
Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Pacifici R et al (2001) Increased production of inflammatory cytokines in patients with silent myocardial ischemia. J Am Coll Cardiol 38:1895–1901. https://doi.org/10.1016/s0735-1097(01)01660-6
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A et al (2002) Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 106:2894–2900
Wang H, Li X, Gong G (2020) Cardiovascular outcomes in patients with co-existing coronary artery disease and rheumatoid arthritis. A Syst Rev Meta-analys Med 99(14):1–11
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R (2010) Extra-articular manifestations in rheumatoid arthritis. Maedica (Bucur) 5(4):286–291 (PMID: 21977172)
Kumar N, Armstrong DJ (2008) Cardiovascular disease – the silent killer in rheumatoid arthritis. Clin Med 8:384–387
Alexander RW (1994) Inflammation and coronary artery disease. N Engl J Med 331:468–469
Pasceri V, Yeh ET (1999) A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation 100(21):2124–2126
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336:973–979
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101:1767–1772
Logstrup BB, Olesen KKW, Masic D, Gyldenkerne C, Thrane PG, Ellingsen T, Botker HE, Maeng M (2020) Impactof rheumatoid arthritis on major cardiovascular events in patients with and without coronary artery disease. Ann Rheum Dis 79:1182–1188
Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS (2014) Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 312(17):1754–1763
Tinggaard AB, Thurah A, Andersen IT, Riis AH, Therkildsen J, Winther S, Hauge EM, Bottcher M (2020) Coronary artery calcification and obstructive coronary artery disease in patients with chest pain: a registry based cross-sectional study. Clin Epidemiol 12:679–689
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, Harvey D (2018) White and The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. J Am Coll Cardiol 72(18):2231–2264
Bozkurt B, Hershberger R, Butler J, Grady KL, Heidenreich PA, Isler ML, Kirklin JK, Weintraub WS (2021) ACC/AHA Key Data Elements and Definitions for Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circulation: Cardiovasc Qual Outcomes 14:e000102. https://doi.org/10.1161/HCQ.0000000000000102
Kim W, Kim EJ (2018) Heart failure as a risk factor for stroke. J Stroke 20(1):33–45. https://doi.org/10.5853/jos.2017.02810
Murphy SJ, Werring DJ (2020) Stroke: causes and clinical features. Medicine (Abingdon) 48(9):561–566. https://doi.org/10.1016/j.mpmed.2020.06.002
Ristl R, Frommlet F, Koch A, Posch M (2016) Fallback tests for co-primary endpoints. Stat Med 35(16):2669–2686. https://doi.org/10.1002/sim.6911
Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73(3):751–754
(2022) Centers for Disease Control and Prevention (CDC). Deaths and Mortality. Accessed: January 13, 2022. https://www.cdc.gov/nchs/fastats/deaths.htm
Taber JM, Leyva B, Persoskie A (2015) Why do people avoid medical care? A qualitative study using national data. J Gen Intern Med 30(3):290–297. https://doi.org/10.1007/s11606-014-3089-1
Czeisler MÉ, Marynak K, Clarke KE et al (2020) Delay or avoidance of medical care because of COVID-19–related concerns — United States. MMWR Morb Mortal Wkly Rep 69:1250–1257. https://doi.org/10.15585/mmwr.mm6936a4
Sheifer SE, Manolio TA, Gersh BJ (2001) Unrecognized myocardial infarction. Ann Intern Med 135(9):801–811. https://doi.org/10.7326/0003-4819-135-9-200111060-00010
Cohn PF, Fox KM, Daly C (2003) Silent myocardial ischemia. Circulation 108:1263–1277
Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Pacifici R et al (2001) Increased production of inflammatory cytokines inpatients with silent myocardial ischemia. J Am Coll Cardiol 38:1895–1901
Li J (2004) Silent myocardial ischemia may be related to inflammatory response. Med Hypotheses 62:252–256
Barber CE, Esdaile JM, Martin LO, Faris P, Barnabe C, Guo S, Lopatina E, Marshall DA (2016 Nov) Gaps in addressing cardiovascular risk in rheumatoid arthritis: assessing performance using cardiovascular quality indicators. J Rheumatol 43(11):1965–1973. https://doi.org/10.3899/jrheum.160241 (Epub 2016 Aug 1 PMID: 27481908)
Eder L, Chandran V, Gladman DD (2014) The Framingham risk score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis 73:1990–1996
Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE (2012) Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 110:420–424
Colaco K, Ocampo V, Ayala AP, Harvey P, Gladman DD, Piguet V, Eder L (2020) Predictive utility of cardiovascular risk prediction algorithms in inflammatory rheumatic diseases: a systematic review. J Rheumatol 47(6):928–938. https://doi.org/10.3899/jrheum.190261
Agca R, Heslinga SC, Rollefstad S et al (2017) EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 76(1):17–28. https://doi.org/10.1136/annrheumdis-2016-209775
Chodara AM, Wattiaux A, Bartels CM (2017) Managing cardiovascular disease risk in rheumatoid arthritis: clinical updates and three strategic approaches. Curr Rheumatol Rep 19(4):16. https://doi.org/10.1007/s11926-017-0643-y
Weijers JM, Semb AG, Rollefstad S et al (2020) Strategies for implementation of guideline recommended cardiovascular risk management for patients with rheumatoid arthritis: results from a questionnaire survey of expert rheumatology centers. Rheumatol Int 40:523–527. https://doi.org/10.1007/s00296-020-04533-4
Weijers JM, Rongen-van Dartel SAA, Hoevenaars DMGMF et al (2018) Implementation of the EULAR cardiovascular risk management guideline in patients with rheumatoid arthritis: results of a successful collaboration between primary and secondary care. Ann Rheum Dis 77(4):480–483. https://doi.org/10.1136/annrheumdis-2017-212392
Acknowledgements
The authors of this study would like to acknowledge Baylor Scott & White Medical Center – Temple, for providing the necessary resources, including access to patient data, IRB reviews with guidance, and a wonderful team of statisticians that made data analysis possible. We also acknowledge the Texas A&M University System for their continued support of research and academia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript, or any part of the work, has not been previously submitted as an abstract, presentation, or for publication elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guerra, J.D., De Santiago, A.B., Reed, S. et al. Cardiology co-management of rheumatoid arthritis patients with coronary artery disease as an intervention reduces hospitalization rates and adverse event occurrence. Clin Rheumatol 41, 3715–3724 (2022). https://doi.org/10.1007/s10067-022-06335-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06335-4