Abstract
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease involving a variety of immune cells, including adaptive T and B cells and innate lymphoid cells (ILCs). Understanding the pathogenic role of these immune cells in RA provides new insights into the intervention and treatment of RA.
Methods
A total of 86 patients with RA (RA group) and 50 healthy controls (HC) were included in the study. The immune cells of CD4+, CD19+ B, NK, Th17, Treg, ILCs, and their subsets (i.e., ILC1s, ILC2s, and ILC3s) were characterized in peripheral blood mononuclear cells by flow cytometry. Cytokines (i.e., IFN-γ, IL-4, IL-10, IL-17A, IL-22, and IL-33) in sera were detected using ELISA. The above immune cells and cytokines were analyzed in patients with different disease activity status and positive ( +) or negative ( −) rheumatoid factor (RF)/anti-citrullinated protein antibodies (ACPA).
Results
Patients with RA had higher percentages of CD4+ T, CD19+ B, Th17, ILC2s, and ILC3s and lower percentages of Treg and ILC1s than HC. Patients with RA had elevated levels of IFN-γ, IL-4, IL-17A, and IL-22 and decreased level of IL-10. Compared with HC, patients with high disease activity had higher percentages of Th17, ILC2s, and ILC3s; lower percentages of ILC1s; and lower level of IL-10. The percentage of Treg cells in remission, low, moderate, and high disease activities decreased, whereas the level of IL-17A increased compared with HC. Furthermore, RF+ or ACPA+ patients exhibited elevated percentages of CD19+ B, ILC2s, and ILC3s and had decreased percentage of ILC1s and Treg cells than HC. The percentage of Th17 cells increased in RF−/ACPA− and RF+/ACPA+ patients. However, the above immune cells between RF or ACPA positive and negative patients were not significantly different.
Conclusion
Th17, Treg, and ILC subset dysregulations are present in patients with RA but may not be associated with conventionally defined seropositive RF and ACPA.
Key Points • Th17, Treg, and ILC subset dysregulations are present in patients with RA but may reflect inflammation rather than specific diseases and stages. • No difference for the distribution of Th17, Treg, and ILC subsets between RF+ and RF− patients and between ACPA+ and ACPA− patients. The screening spectrum of RF and ACPA serology should be expanded to elucidate the role of immune cells in RA pathogenesis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammation characterized by the infiltration of inflammatory cells in the joint synovium, causing joint destruction, disability, and pathological change of extra-articular sites. Recently, the pathogenesis of RA demonstrated that the aberrant activation of innate and adaptive immune cells by the complex interaction between genetic and environmental factors, which disrupts the immune tolerance, causes the presentation of autoantigen and inflammatory cytokine secretion [1, 2]. Various immune cells have been found in the joint of patients with RA, especially CD4+ T cells, B cells, and macrophages [3, 4]. Cytokines produced by macrophage activate fibroblast-like synoviocytes and osteoclasts, promote bone destruction, and drive CD4+ T cell polarization and B cell activation [3, 5]. Th1 cells are originally thought to be one of the main pathogenic factors of RA. Recently, IL-17 and Th17 cells have been thought to have critical roles in RA pathogenesis. CD14+ monocytes from the inflamed joints of patients with RA can potently induce Th17 cells instead of Th1 cells [6]. Furthermore, B cells/plasma cells produce autoantibodies especially rheumatoid factors (RF) and anti-citrullinated peptide antibodies (ACPAs), which promote T cell activation [7, 8]. Thus, autoreactive T and B cells play a critical role in the pathogenesis of RA.
In addition to B and T cells, innate lymphoid cells (ILCs) are considered to play an important role in the pathogenesis of RA. Helper-like lymphoid cells, as the most prominent ILCs, are usually classified into ILC1, ILC2, and ILC3 subsets and are counterpart of Th cells [9]. Similar to Th cells, ILC1s require T-bet and produce IFN-γ. ILC2s express GATA3 and secrete IL-4, IL-5, and IL-13. ILC3s express RORγt and secrete IL-17 and IL-22. ILCs can be a bridge between innate and adaptive immunity, thereby mediating inflammatory responses, including RA [9]. Takaki-Kuwahara and colleagues reported that CCR6+ILC3s increases in joints of arthritic mice and patients with RA, and CCR6+ILC3s may participate in the development of RA by producing IL-17 and IL-22 [10]. However, Yang and colleagues found that stable patients with RA have increased proportions of ILC2s and reduced percentages of ILC1s and ILC3s. The percentages of ILC1s and ILC2s are increased in mice with collagen-induced arthritis (CIA), indicating that the dysregulation of ILCs participates in the development of RA and CIA [11]. Thus, we hypothesized that intrinsic and acquired immune cell imbalances, accompanied with the production of inflammatory cytokines, are associated with the development of RA. An improved understanding of the role and function of the abovementioned innate and adaptive immune cells, especially ILC subsets, in the pathogenesis of RA can provide new interventions for the treatment of RA.
In this study, we analyze the immune cells especially ILCs and their three subsets in the peripheral blood of patients with RA by flow cytometry. We demonstrate that the proportions of CD4+T, CD19+B, Th17, ILC2, and ILC3 cells in the peripheral blood of patients with RA increase, and the proportions of Treg and ILC1 cells decrease. The percentages of CD19+ B, Th17, ILC2s, and ILC3s increase in RF- (RF+) or ACPA-positive (ACPA+) patients. The percentages of ILC1s and Treg cells decrease in RF+ or ACPA+ patients. The above immune cells have not been found with a significant difference between RF or ACPA seropositive and seronegative patients, suggesting that the dysregulation of these immune cells may not be associated with conventionally defined seropositive RF and ACPA.
Materials and methods
Patients
A total of 86 patients with RA fulfilling the criteria of the American College of Rheumatology [12] and 50 age- and sex-matched healthy controls were enrolled into this study. Disease activity was assessed using the 28-joint disease activity score (DAS28) [13], and all patients were free of infectious diseases, malignant diseases, cardiovascular complaints, or other inflammatory diseases. The characteristics of patients with RA and healthy controls are summarized in Table S11. None had been taking disease modifying anti-rheumatic drugs. On the basis of DAS28 values, patients were classified as remission (DAS28 < 2.6, n = 25), low-disease activity (2.6 ≤ DAS28 ≤ 3.2, n = 19), moderate-disease activity (3.2 < DAS28 ≤ 5.1, n = 27), and high-disease activity (DAS28 > 5.1, n = 15) groups as previously described [14]. Informed consent was obtained from all patients and healthy controls, and the study was approved by the ethics committee of the Affiliated Hospital of Jiangsu University (Permit Number: SWYXLL20191119-15) and performed in accordance with the Helsinki Declaration on ethical principles for medical research involving human participants.
Blood sample preparation
Peripheral blood mononuclear cells (PBMC) were isolated from patients with RA and HC by the Ficoll–Hypaque (Solarbio, Beijing, China) density gradient centrifugation. Then, PBMC were performed immunophenotyping by flow cytometry. Serum was separated from the specimens and stored at − 80 °C until use for cytokine determination by enzyme-linked immunosorbent assay (ELISA). ACPA was measured using an anti-cyclic citrullinated peptide assay ELISA kit (Fuchunkexin, Shanghai, China) with an upper limit of normal of 25 IU/mL. ACPA > 25 IU/mL was considered positive (ACPA+), and ACPA < 25 IU/mL was considered negative (ACPA−) as previously described [15]. The C-reactive protein (CRP) and IgM-RF were measured using the Beckman Coulter IMMAGE800 (Beckman Coulter). RF > 20 IU/mL was considered positive (RF+), whereas RF < 20 IU/mL was considered negative (RF−) [15].
Flow cytometry
For the analysis of Th17 cells, PBMC were suspended at a density of 1 × 106 cells/mL in complete culture medium (RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum) for 5 h in the presence of phorbol myristate acetate (25 ng/mL) and brefeldin A (1 μg/mL) at 37 °C and 5% CO2. Cells were then incubated with human fluorescein isothiocyanate (FITC)–anti-CD4 mAb, washed, fixed, and permeabilized with Cytofix/Cytoperm (BD PharMingen, San Diego, CA, USA). Cells were then intracellularly stained with phycoerythrin (PE)–anti-IL-17A or PE-conjugated rat IgG1 (isotype control) for 30 min at 4 °C based on previous publications [16].
For the analysis of Treg cells, PBMC were stained with human FITC–anti-CD4 mAb, and APC–anti-CD25 mAb, fixed, permeabilized with Cytofix/Cytoperm, and intracellularly stained with PE–anti-Foxp3 or PE–IgG2a rat IgG control antibody in accordance with the manufacturer’s instructions.
For the analysis of ILCs, the following antibodies were used to quantify ILCs based on previous publications [17]: eFluor450-conjugated anti-CD3 (eBioscience), allophycocyanin (APC)–Cyanine7-conjugated anti-CD19 (eBioscience), PE–Cyanine7-conjugated anti-CD56 (NCAM, eBioscience), FITC-conjugated anti-CD127 (eBioscience), APC-conjugated anti-CD117 (cKit, eBioscience), and PE-conjugated anti-CD294 (CRTH2, eBioscience). ILCs were defined as cells within the lymphocyte gate on the scatter plot that were single cells, lineage negative (i.e., CD3−CD19−CD56−) and CD127 positive. ILCs were subsequently classified as ILC1s (Lin−CD127+CRTH2−c-Kit−), ILC2s (Lin−CD127+CRTH2+), or ILC3s (Lin−CD127+CRTH2−c-Kit+) based on the markers CD117 (c-Kit) and CD294 (CRTH2). Samples were analyzed using the BD FACSCanto flow cytometer (BD Biosciences) and Flowjo Software (Tree Star).
ELISA
The analysis of cytokines in serum from patients with RA and HC was conducted using human ELISA kits (Multi Sciences, Hangzhou, China). All assays were performed in accordance with the manufacturer’s instructions. Six cytokines, i.e., human interferon (IFN)-γ, interleukin (IL)-4, IL-10, IL-17A, IL-22, and IL-33, were measured.
Statistical analyses
Normally distributed data are presented as mean ± SEM. Student’s t-test or one-way ANOVA test were used for determining significant differences between groups. Non-normally distributed data were presented as median (interquartile range) and analyzed using the Mann–Whitney U or Kruskal–Wallis test. P values < 0.05 were considered significant. Data were analyzed using the GraphPad Prism version 8.0.
Results
Elevation of circulating CD4+ T, CD19+ B, Th17, ILC2s, and ILC3s percentages and reduction of Treg and ILC1s percentages in patients with RA
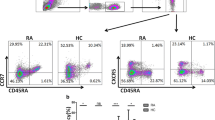
Blood lymphocytes from patients with RA and HC were analyzed by flow cytometry. Consistent with the participation of CD4+T and B cells in the pathogenesis of RA [18], the percentages of CD4+ T and CD19+ B cells in patients with RA were remarkably increased compared with those in HC (Fig. 1). Furthermore, consistent with our results and others described previously [19, 20], compared with those in HC, the percentage of Th17 cells (0.46 ± 0.05, P = 0.0025) in patients with RA increased, whereas the percentage of Treg cells (1.47 ± 0.18, P < 0.0001) decreased (Fig. 1). No significant difference was observed between NK cells (14.35 ± 1.32, P = 0.7725) and total ILCs (2.62 ± 0.30, P = 0.89) between patients with RA and HC (Figure S1 for gating strategy of ILCs). However, patients with RA had increased percentages of ILC2s (38.41 ± 2.48, P = 0.0002) and ILC3s (24.22 ± 1.62, P = 0.0018) and decreased percentage of ILC1s (40.09 ± 1.93, P = 0.037) compared with HC (Fig. 1). Thus, B and CD4+T cells especially Th17/Treg and ILC subsets imbalance contributed to the development of the inflammatory process of RA.
Flow cytometry analysis of circulating lymphocyte subsets in RA patients and HC. a CD4+ T cells, b CD19+ B cells, c CD4+IL-17+ Th17 cells, d CD4+CD25+Foxp3+ Treg cells, e CD3−CD56+ NK cells, f CD3−CD19−CD56−CD127+ ILCs, and g Lin−CD294−CD117− ILC1s, Lin−CD294+ ILC2s, and Lin−CD294−CD117+ ILC3s. Data are representative of the experiments. h The percentage of CD4+ T cells, i CD19+ B cells, j Th17 cells, k Treg cells, l NK cells, m total ILCs, n ILC1s, o ILC2s, and p ILC3s in each group. Results are presented as mean ± SEM. *P < 0.05,**P < 0.01, ***P < 0.001
Elevation of IFN-γ, IL-4, IL-17A, and IL-22 levels and reduction of IL-10 level in patients with RA
Considering the important role of cytokines in the pathogenesis of RA, we analyzed the cytokine level in the sera of patients with RA and HC. As shown in Fig. 2, compared with that of HC, the sera of patients with RA was observed with higher levels of IFN-γ (14.98 ± 1.22, P = 0.0042), IL-4 (10.62 ± 1.43, P = 0.0006), IL-17A (34.27 ± 5.30, P < 0.0001), and IL-22 (197.00 ± 27.13, P = 0.0483) and lower level of IL-10 (5.43 ± 0.50, P = 0.043). However, the level of IL-33 (191.90 ± 10.96, P = 0.4814) between patients with RA and HC was not statistically different (Fig. 2).
Elevation of circulating Th17, ILC2s, and ILC3s percentages and reduction of Treg and ILC1s percentages in patients with RA and high disease status
To explore the relationship between circulating immune cells and disease activity, we analyzed the immune cell phenotype in different disease activity status of patients with RA. The clinical characteristics of different disease activity status are shown in Figure S2. Compared with the HC, groups with low (30.74 ± 3.47, P = 0.0002), moderate (54.56 ± 6.69, P < 0.0001), and high disease activities displayed higher ESR (77.00 ± 17.43, P < 0.0001). Groups with low (30.74 ± 3.47, P = 0.0490), moderate (54.56 ± 6.69, P < 0.0001), and high disease activities (77.00 ± 17.43, P = 0.0082) also showed higher ESR level compared with the remission group. CRP (12.46 ± 1.80, P < 0.0001) and RF (192.9 ± 35.58, P < 0.0001) levels were higher in patients with RA regardless of disease activity status compared with those in HC (Figure S2). No statistical difference in CRP and RF levels was observed among remission group and different disease activity groups. However, the percentages of Th17 cells in moderate- (0.63 ± 0.12, P = 0.0138) and high-disease activity groups (0.67 ± 0.12, P = 0.0028) were significantly increased compared with those in the HC (Fig. 3). Furthermore, the high-disease activity group had higher levels of ILC2s (48.82 ± 3.47, P < 0.0001) and ILC3s (30.30 ± 3.76, P = 0.0249) and lower level of ILC1s (33.71 ± 3.46, P = 0.0346) compared with HC (Fig. 3). The percentage of Treg cells in remission (1.73 ± 0.30, P = 0.0104), low (1.58 ± 0.30, P = 0.0034), moderate (1.08 ± 0.19, P < 0.0001), and high disease activities (0.80 ± 0.13, P < 0.0001) also decreased compared with HC (Fig. 3). No significant difference in the proportion of total ILCs (2.62 ± 0.30, P = 0.89) was observed among the healthy control group and different disease activity groups. The distributions of Th17, Treg, and ILC subsets were not different among the different disease stages.
Flow cytometry analysis of circulating lymphocyte subsets in HC and RA patients with different disease activity status. a CD4+IL-17+ Th17 cells, b CD4+CD25+Foxp3+ Treg cells, c CD3−CD19−CD56−CD127+ ILCs, and d Lin−CD294−CD117− ILC1s, Lin−CD294+ ILC2s, and Lin−CD294−CD117+ ILC3s. Data are representative of the experiments. e The percentage of Th17 cells, f Treg cells, g total ILCs, h ILC1s, i ILC2s, and j ILC3s in each group. Results are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001
Elevated IL-17A level and decreased IL-10 level in the serum of patients with high disease activity
Given that cytokines are the key inflammatory mediators in patients with RA, we analyzed the cytokine secretion in sera from different disease activity status of patients with RA. As shown in Fig. 4, compared with healthy controls, patients in the remission group (29.17 ± 6.32, P = 0.0112) and low- (31.82 ± 6.50, P = 0.0118), moderate- (37.14 ± 8.17, P = 0.0020), and high-disease activity groups (40.59 ± 10.55, P = 0.0032) had increased IL-17A level. However, the IL-10 level (3.26 ± 0.82, P = 0.0233) was decreased in patients with high disease activity. Furthermore, the levels of IFN-γ, IL-4, IL-22, and IL-33 were not statistically different among HC and patients with RA and different disease activity status.
Elevation of CD19+ B, Th17, ILC2s, and ILC3s percentages and reduction of Treg and ILC1s percentages in RF+ patients
RF is the first autoantibody and is related to high disease activity [21]. Thus, we analyzed the immune cell phenotype and cytokine secretion in RF+ and RF− patients. As shown in Fig. 5, RF+ patients had increased percentages of CD19+ B (9.44 ± 0.50, P = 0.0354), ILC2s (38.64 ± 2.72, P = 0.0009), and ILC3s (23.87 ± 1.72, P = 0.0352) and decreased percentage of Treg (1.32 ± 0.15, P < 0.0001) and ILC1s (38.06 ± 2.02, P = 0.0396) compared with HC (Fig. 5). RF+ (0.45 ± 0.06, P = 0.0318) and RF− (0.57 ± 0.08, P = 0.0164) patients had increased Th17 cell percentage, and the levels of IL-17A of RF+ (50.21 ± 9.73, P = < 0.0001) and RF− (32.98 ± 6.11, P = 0.0392) patients were higher compared with HC. However, no significant difference in the proportion of CD4+ T, Treg, NK, and total ILCs was observed among the healthy control, RF+, and RF− groups. The levels of IFN-γ, IL-4, IL-10, IL-22, and IL-33 were not statistically different among HC and RF+ or RF− patients (Figure S3). Furthermore, the above cells and cytokines displayed no significant difference between RF+ and RF− patients.
Flow cytometry analysis of circulating lymphocyte subsets in HC, RF−, and RF+ RA patients. a CD4+ T cells, b CD19+ B cells, c CD4+IL-17+ Th17 cells, d CD4+CD25+Foxp3+ Treg cells, e CD3−CD56+ NK cells, f CD3−CD19−CD56−CD127+ ILCs, and g Lin−CD294−CD117− ILC1s, Lin−CD294+ ILC2s, and Lin−CD294−CD117+ ILC3s. Data are representative of the experiments. h The percentage of CD4+ T cells, i CD19+ B cells, j Th17 cells, k Treg cells, l NK cells, m total ILCs, n ILC1s, o ILC2s, and p ILC3s in each group. Results are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Elevation of CD19+ B, Th17, ILC2s, and ILC3s percentages and reduction of Treg and ILC1s percentages in ACPA+ patients
ACPA is another main antibody and is related to the disease progression of patients with RA [22, 23]. Thus, the immune cell phenotype and cytokine secretion in ACPA+ and ACPA− patients were analyzed. As shown in Fig. 6, ACPA+ patients showed increase percentages of CD19+ B (10.21 ± 0.61, P = 0.0060), ILC2s (39.15 ± 2.70, P = 0.0445), and ILC3s (24.86 ± 1.89, P = 0.0130) and decreased percentages of Treg (1.33 ± 0.16, P < 0.0001) and ILC1s (38.16 ± 1.84, P = 0.0391) compared with HC (Fig. 6). ACPA+ (0.47 ± 0.07, P = 0.0445) and ACPA− patients (0.44 ± 0.06, P = 0.0364) all displayed the increased percentage of Th17 cells compared with HC. Serum IL-17A in ACPA+ (33.29 ± 3.75, P < 0.0001) and ACPA− (32.20 ± 6.47, P = 0.0408) patients were higher than those in HC (Figure S4).
Flow cytometry analysis of circulating lymphocyte subsets in HC, ACPA−, and ACPA+ RA patients. a CD4+ T cells, b CD19+ B cells, c CD4+IL-17+ Th17 cells, d CD4+CD25+Foxp3+ Treg cells, e CD3−CD56+ NK cells, f CD3−CD19−CD56−CD127+ ILCs, and g Lin−CD294−CD117− ILC1s, Lin−CD294+ ILC2s, and Lin−CD294−CD117+ ILC3s. Data are representative of the experiments. h The percentage of CD4+ T cells, i CD19+ B cells, j Th17 cells, k Treg cells, l NK cells, m total ILCs, n ILC1s, o ILC2s, and p ILC3s in each group. Results are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
RA is a chronic inflammatory disease. Although the pathogenic mechanisms of RA have not been fully elucidated, the aberrant activation of innate and adaptive immune system with consequent production of autoantibodies and inflammatory cytokines participates in the development of RA [18]. In this setting, various immune cells, such as T cells, B cells, and other innate immune cells, form a complex network and produce proinflammatory cytokines, which eventually lead to joint damage [3]. In addition to adaptive T and B cells, the ILCs involved in inflammatory and autoimmune responses have been confirmed to participate in the pathogenesis of RA [3]. Rauber and colleagues demonstrated that patients with RA and in remission have high numbers of IL-9+ILC2s [24]. In the present study, we demonstrated that patients with RA have upregulated percentages of circulating CD4+ T, CD19+ B, Th17, ILC2s, and ILC3s and downregulated percentages of Treg and ILC1s. Increased Th17 and decreased Treg frequencies in patients with RA in the present study are consistent with previous studies [19, 20]. In contrast to the reduction of ILC1s and increase of ILC3s in PBMC of patients with RA, Leijten and colleagues reported that the frequencies of ILC1s increase, and ILC3s decrease in the synovial fluid (SF) of patients with RA [25]. ILCs initially serve as tissue “sentinel,” which can produce a variety of cytokines upon activation in an inflammatory environment. Furthermore, ILCs initiate helper T cell responses [26], and activated Th cells produce proinflammatory cytokines in chronic inflammation. Consistent with increased ILC2s and ILC3s frequencies, IL-4 and IL-17 levels in the sera of patients with RA are also increased. However, whether IL-4 is secreted from ILC2s or Th2 and whether IL-17 is produced from ILC3s or Th17 are unclear. Inconsistent with the decrease in ILC1s percentage, the IFN-γ level in the sera of patients with RA is increased. Th1 cells and IFN-γ have been suggested to contribute to the pathogenesis in RA[27], and IL-4 and other Th2 cytokines downregulate the inflammatory processes in RA [28, 29]. However, SF from patients with early arthritis display increased Th2 cytokines, such as IL-4 and IL-13, but not Th1-related IFN-γ [30]. Furthermore, Kokkonen and colleagues reported that the sera from patients after the development of RA show increases in Th1-related IFN-γ, Th2-related IL-4 and IL-13, and immune regulation IL-10 [31]. In addition, IFN-γ may be derived from Th17 and CD8+ T cells (Tc1 cells) in patients with RA [27, 32] although we have not identified IFN-γ-producing cells. Consistent with the increase in Th17 cells, the levels of IL-22, a Th17-related-effector molecule, in the sera of patients with RA increase [32]. However, the IL-10 level in the sera of patients with RA in this study is decreased, which is contrary to other studies in which IL-10 is elevated in blood and SF [31, 33]. The IL-10 level in this study is consistent with another study, demonstrating that the IL-10 concentration in culture supernatants from PBMCs and SFMCs in patients with RA is lower than that in healthy controls [34]. The differences in immune cells and cytokines from patients with RA may be related to the difference in disease populations and stages. The dysregulation of these immune cells and cytokines may reflect inflammation rather than specific diseases.
Consistent with the view that Th17 and IL-17 have dominant pathogenic roles in RA [32], the Th17 cell frequency is increased in patients with high disease activity, whereas the Treg percentage is decreased. These results are consistent with the findings that active patients with RA have high expression of RORc mRNA and low expression of FoxP3 mRNA than that of inactive patients and healthy controls [35], because FoxP3 and RORc are the lineage-specific transcription factors of Treg and Th17 cells. In contrast to stable patients who have increased ILC1s and decreased ILC2s [11], patients with high disease activity have decreased ILC1s and increased ILC2s and ILC3s. Although ILC2s is thought to be involved in the resolution of inflammation in RA [24, 36], GM-CSF-producing ILC2s is reported to play a pathogenic role in the development of arthritis [37]. Takaki-Kuwahara A et al. revealed that the proportion of ILC1s in SF is negatively correlated with tender (TJC) and swollen (SJC) joint counts, but NKp44+ILC3s is positively correlated with TJC and SJC, suggesting that ILC3s aggravate the inflammation in patients with RA [10]. Activated ILCs are also important sources of inflammatory cytokines. High frequencies of Th17 and ILC3s are consistent with high levels of IL-17 in patients with RA and high disease activity. Furthermore, a low level of IL-10 is consistent with low frequencies of Treg cells in patients with RA and high disease activity. IL-10 is produced by Treg, Th1, Th2, and other cells [34]. This finding supports the notion that active patients may exhaust IL-10 due to continuous against inflammation [38]. Furthermore, these results are consistent with a previous study demonstrating that as inflammation increases, patients reveal low percentage of CD4+CD25+ Treg cells [38]. Thus, the imbalance in the Th17/Treg and ILC subsets involving the production of pro-/anti-inflammatory cytokines is associated with the development and/or progression of RA.
Patients positive for RF and/or ACPA are usually considered to be seropositive and considered to be correlated with high disease activity and poor clinical outcome [39]. RF+ and ACPA+ patients have increased percentages of ILC2s and ILC3s and decreased percentage of Treg and ILC1s relative to healthy controls. The above ILC subsets are not significantly different between RF+ and RF− or ACPA+ and ACPA− patients. Compared with HC, RF+, RF−, ACPA+, and ACPA− patients have increased Th17 cells and IL-17A levels. Compared with HC, RF+ or ACPA+ patients have decreased Treg cells. However, the frequencies of Th17 and Treg cells show no statistical difference between RF+ and RF− patients or between ACPA+ and ACPA− patients. Although ACPA has been reported to stimulate macrophages to produce cytokines through the formation of immune complexes and participate in the pathogenesis of RA [40], we do not observe differences in the above immune cells and cytokines between ACPA+ and ACPA− patients. No difference for the distribution of Th17 and Treg cells is observed between ACPA+ and ACPA− patients, and this finding is consistent with a previous study [41]. However, Paulissen et al. reported that ACPA+ patients show high percentages of Th22, Th17.1, and CCR4/CXCR3 double-positive cells, which are gated from CCR6+ population and suggested that CCR6 + Th cell differentiates ACPA+ patients from ACPA− patients [41]. Identifying the relationship between immune cell populations and autoantibodies may require identification markers. However, ACPA+ and RF+ as “seropositive” are commonly determined by anti-CCP2 IgG and IgM RF, respectively. The CCP2 test does not capture all ACPA because many proteins, such as citrullinated fibrinogen, collagen type II, tenascin C, α-enolase, vimentin, and histones, are recently described as ACPA targets [42]. Rönnelid and colleagues demonstrated that 16% of anti-CCP2-negative are ACPA+ in patients with RA by using a multiplex citrullinated peptide array [43]. Furthermore, the IgA isotype of RF and anti-CCP antibody has been found to be superior to IgM- or IgG-RF and anti-CCP response in mucosal inflammation and is closely associated with the development of RA [44,45,46]. Thus, Reed E et al. reported that ACPA− or RF− is not truly a seronegative subset in patients with RA [42]. ACPA fine specificities and IgA/IgG RF for anti-CCP2-/IgM RF − patients should be screened, which may contribute to early diagnosis and therapy for these “seronegative” patients [42]. Thus, immune cell and cytokine dysregulation should be analyzed on the basis of a broad spectrum of RF and ACPA serology. However, this study had limitations because the results were from a limited number of patients, so the sample size needs to be expanded for validation.
Conclusion
In conclusion, we demonstrated that patients with RA have a variety of immune cell disorders, such as CD4+T, CD19+B, Th17, Treg, and ILC subsets, but these immune cell dysregulations may not be related to RF and ACPA autoantibodies. Further studies are necessary to unravel the roles of immune cell populations precisely in the development of RA by increased identification markers or screening a broad spectrum of RF and ACPA serology.
Data availability
All data generated or analyzed during this study are included in this article.
References
Deane KD, Holers VM (2021) Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift. Arthritis Rheumatol 73(2):181–193. https://doi.org/10.1002/art.41417
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS et al (2018) Rheumatoid arthritis Nat Rev Dis Primers 4:18002. https://doi.org/10.1038/nrdp.2018.1
Roberts CA, Dickinson AK, Taams LS (2015) The interplay between monocytes/macrophages and CD4(+) T cell subsets in rheumatoid arthritis. Front Immunol 6:571. https://doi.org/10.3389/fimmu.2015.00571
Tu J, Huang W, Zhang W, Mei J, Zhu C (2021) A tale of two immune cells in rheumatoid arthritis: the crosstalk between macrophages and T cells in the synovium. Front Immunol 12:655477. https://doi.org/10.3389/fimmu.2021.655477
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118(11):3537–3545. https://doi.org/10.1172/JCI36389
Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW et al (2009) In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci U S A 106(15):6232–6237. https://doi.org/10.1073/pnas.0808144106
Kerkman PF, Fabre E, van der Voort EI, Zaldumbide A, Rombouts Y, Rispens T et al (2016) Identification and characterisation of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis. Ann Rheum Dis 75(6):1170–1176. https://doi.org/10.1136/annrheumdis-2014-207182
Volkov M, van Schie KA, van der Woude D (2020) Autoantibodies and B cells: the ABC of rheumatoid arthritis pathophysiology. Immunol Rev 294(1):148–163. https://doi.org/10.1111/imr.12829
Guia S, Narni-Mancinelli E (2020) Helper-like innate lymphoid cells in humans and mice. Trends Immunol 41(5):436–452. https://doi.org/10.1016/j.it.2020.03.002
Takaki-Kuwahara A, Arinobu Y, Miyawaki K, Yamada H, Tsuzuki H, Irino K et al (2019) CCR6+ group 3 innate lymphoid cells accumulate in inflamed joints in rheumatoid arthritis and produce Th17 cytokines. Arthritis Res Ther 21(1):198. https://doi.org/10.1186/s13075-019-1984-x
Yang F, Luo X, Zhu W, Li J, Zheng Z, Zhu P (2021) Dysregulation of innate lymphoid cells in patients with active rheumatoid arthritis and mice with collagen-induced arthritis. Mediators Inflamm 2021:1915068. https://doi.org/10.1155/2021/1915068
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324. https://doi.org/10.1002/art.1780310302
Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1): 44-48. https://doi.org/10.1002/art.1780380107
Guderud K, Sunde LH, Flam ST, Maehlen MT, Mjaavatten MD, Norli ES et al (2021) Methotrexate treatment of newly diagnosed RA patients is associated with DNA methylation differences at genes relevant for disease pathogenesis and pharmacological action. Front Immunol 12:713611. https://doi.org/10.3389/fimmu.2021.713611
Hernandez-Breijo B, Brenis CM, Plasencia-Rodriguez C, Martinez-Feito A, Novella-Navarro M, Pascual-Salcedo D et al (2021) Methotrexate reduces the probability of discontinuation of TNF inhibitors in seropositive patients with rheumatoid arthritis. A Real-World Data Analysis Front Med (Lausanne) 8:692557. https://doi.org/10.3389/fmed.2021.692557
Wang Y, Su R, Li B, Guo Q, Hu F, Yu X et al (2021) Reduction of peripheral regulatory T cells in active rheumatoid arthritis patients with coronary artery disease. BMC Immunol 22(1):76. https://doi.org/10.1186/s12865-021-00466-0
Chevalier MF, Trabanelli S, Racle J, Salome B, Cesson V, Gharbi D et al (2017) ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest 127(8):2916–2929. https://doi.org/10.1172/JCI89717
Calabresi E, Petrelli F, Bonifacio AF, Puxeddu I, Alunno A (2018) One year in review 2018: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 36(2):175–184 (PMID: 29716677)
Dong L, Wang X, Tan J, Li H, Qian W, Chen J et al (2014) Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J Cell Mol Med 18(11):2213–2224. https://doi.org/10.1111/jcmm.12353
Paradowska-Gorycka A, Wajda A, Romanowska-Prochnicka K, Walczuk E, Kuca-Warnawin E, Kmiolek T et al (2020) Th17/Treg-related transcriptional factor expression and cytokine profile in patients with rheumatoid arthritis. Front Immunol 11:572858. https://doi.org/10.3389/fimmu.2020.572858
Aletaha D, Alasti F, Smolen JS (2013) Rheumatoid factor determines structural progression of rheumatoid arthritis dependent and independent of disease activity. Ann Rheum Dis 72(6):875–880. https://doi.org/10.1136/annrheumdis-2012-201517
Catrina A, Krishnamurthy A, Rethi B (2021) Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 7(1):e001228. https://doi.org/10.1136/rmdopen-2020-001228
Sun M, Rethi B, Krishnamurthy A, Joshua V, Circiumaru A, Hensvold AH et al (2019) Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Ann Rheum Dis 78(12):1621–1631. https://doi.org/10.1136/annrheumdis-2018-214967
Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T et al (2017) Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 23(8):938–944. https://doi.org/10.1038/nm.4373
Leijten EF, van Kempen TS, Boes M, Michels-van Amelsfort JM, Hijnen D, Hartgring SA et al (2015) Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol 67(10):2673–2678. https://doi.org/10.1002/art.39261
Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM et al (2014) MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41(2):283–295. https://doi.org/10.1016/j.immuni.2014.06.016
Berner B, Akca D, Jung T, Muller GA, Reuss-Borst MA (2000) Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol 27(5):1128–1135 (PMID: 10813277)
Chen Z, Bozec A, Ramming A, Schett G (2019) Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 15(1):9–17. https://doi.org/10.1038/s41584-018-0109-2
Iwaszko M, Bialy S, Bogunia-Kubik K (2021) Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells 10(11). https://doi.org/10.3390/cells10113000
Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN et al (2005) Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 7(4):R784-795. https://doi.org/10.1186/ar1733
Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S (2010) Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 62(2):383–391. https://doi.org/10.1002/art.27186
van Hamburg JP, Tas SW (2018) Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J Autoimmun 87:69–81 (PMID: 29254845)
Keystone E, Wherry J, Grint P (1998) IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am 24(3):629–639. https://doi.org/10.1016/s0889-857x(05)70030-2
Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML et al (2010) IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett 127(2):150–156. https://doi.org/10.1016/j.imlet.2009.10.006
Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J et al (2018) Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann Rheum Dis 77(11):1644–1652. https://doi.org/10.1136/annrheumdis-2018-213511
Omata Y, Frech M, Primbs T, Lucas S, Andreev D, Scholtysek C et al (2018) Group 2 innate lymphoid cells attenuate inflammatory arthritis and protect from bone destruction in mice. Cell Rep 24(1):169–180. https://doi.org/10.1016/j.celrep.2018.06.005
Hirota K, Hashimoto M, Ito Y, Matsuura M, Ito H, Tanaka M et al (2018) Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity 48(6): 1220–1232 e1225. https://doi.org/10.1016/j.immuni.2018.04.009
Sempere-Ortells JM, Perez-Garcia V, Marin-Alberca G, Peris-Pertusa A, Benito JM, Marco FM et al (2009) Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28. Autoimmunity 42(8):636–645. https://doi.org/10.3109/08916930903061491
Pongratz G, Frieser R, Brinks R, Schneider M, Hartung W, Fleck M et al (2020) Association between autoantibody level and disease activity in rheumatoid arthritis is dependent on baseline inflammation. Clin Exp Rheumatol 38(4):691–698 (PMID: 31858962)
Kondo N, Kuroda T, Kobayashi D (2021) Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci 22(20). https://doi.org/10.3390/ijms222010922
Paulissen SM, van Hamburg JP, Davelaar N, Vroman H, Hazes JM, de Jong PH et al (2015) CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther 17:344. https://doi.org/10.1186/s13075-015-0800-5
Reed E, Hedstrom AK, Hansson M, Mathsson-Alm L, Brynedal B, Saevarsdottir S et al (2020) Presence of autoantibodies in “seronegative” rheumatoid arthritis associates with classical risk factors and high disease activity. Arthritis Res Ther 22(1):170. https://doi.org/10.1186/s13075-020-02191-2
Ronnelid J, Hansson M, Mathsson-Alm L, Cornillet M, Reed E, Jakobsson PJ et al (2018) Anticitrullinated protein/peptide antibody multiplexing defines an extended group of ACPA-positive rheumatoid arthritis patients with distinct genetic and environmental determinants. Ann Rheum Dis 77(2):203–211. https://doi.org/10.1136/annrheumdis-2017-211782
Arlestig L, Mullazehi M, Kokkonen H, Rocklov J, Ronnelid J, Dahlqvist SR (2012) Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann Rheum Dis 71(6):825–829. https://doi.org/10.1136/annrheumdis-2011-200668
Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J et al (2011) Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther 13(1):R13. https://doi.org/10.1186/ar3237
Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF et al (2013) Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 65(10):2545–2554. https://doi.org/10.1002/art.38066
Funding
This work was supported by a grant from the National Natural Science Foundation of China (81871243), the Key Research and Development Programs of Jiangsu Province (BE2017697), the Six Talent Peaks of Jiangsu Province (WSN-009), “LiuGeYi” Projects of Jiangsu Province (LGY2016055), “XueDiJiFang” Projects of Jiangsu Province (× 201812), and the Affiliated Hospital of Jiangsu University (jdfyRC2015010).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: TW, JBR, and XFW. Performed the experiments: TW, JBR, WQS, and FX. Analyzed the data: LYD, DQF, and JHM. Contributed reagents/materials/analysis tools: YS and CMM. Wrote the paper: TW, JBR, and XFW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethics committee of the Affiliated Hospital of Jiangsu University (Permit Number: SWYXLL20191119-15) and performed in accordance with the Helsinki Declaration on ethical principles for medical research involving human participants.
Consent to participate
Informed consent was obtained from all individual participants included in the study. The authors confirm that all patient/personal identifiers have been removed or disguised in accordance with ethical requirements.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, T., Rui, J., Shan, W. et al. Imbalance of Th17, Treg, and helper innate lymphoid cell in the peripheral blood of patients with rheumatoid arthritis. Clin Rheumatol 41, 3837–3849 (2022). https://doi.org/10.1007/s10067-022-06315-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06315-8