Abstract
Objective
We preformed this retrospective study of clinical manifestation, imaging feature, and mutations to describe joint involvement in X-linked agammaglobulinemia (XLA) patients, aimed to provide recommendation for physicians.
Methods
A total number of 98 XLA patients who have been diagnosed between January 2000 and February 2020 were enrolled and grouped based on whether they developed arthritis and analyzed for the clinical, imaging, and gene mutation data using the t test or the Mann–Whitney test.
Results
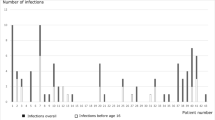
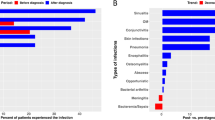
Forty-five out of 98 patients (45.9%) had joint involvement, 40.8% had symptom prior to the diagnosis of XLA, and 54.1% had no articular symptom. Patients with joint involvement had a higher median diagnostic age of XLA and initial IgG level than patients without it, while their intravenous immunoglobulin was lower (p < 0.05). Knee, hip, and ankle were the most frequent joint, and oligoarthritis (≦ 4 joints) was more common than polyarthritis (88.9% vs 11.1%). Red and tenderness were the most frequent clinical symptoms (80%) with 24.4% reporting limited activity and 8.9% reporting deformity. Imaging data collected from 32 patients indicated that joint effusion (53.3%), synovitis (15.5%), and swollen soft tissue (15.5%) were the most common feature. Seventeen patients were treated by antibiotics plus intravenous immunoglobulin (IVIG) with an effective rate of 70.6%, and 28 patients only received IVIG with an effective rate of 67.9%. In comparison to patients without arthritis who have higher frequency nonsense and frameshift mutation, patients with arthritis had a higher incidence of missense mutation (p < 0.05).
Conclusion
High prevalence of arthritis among X-linked agammaglobulinemia patients and subsequent progression through IVIG replacement therapy highlight the importance of timely diagnosis and better management of these patients. Our finding indicated a potential correlation between genotype and phenotype, and further research on the mechanism of arthritis in XLA patients could increase physicians’ awareness and improve patients’ prognosis.
Key Points • This study described the feature of arthritis in XLA patients and indicated a potential correlation between this complication and genotype. |





Similar content being viewed by others
Availability of data and material
The datasets are available from the authors upon reasonable request and with permission of the Institutional Review Board of Children’s Hospital, Chongqing Medical University.
Code availability
The SPSSAU project (2021). SPSSAU. (Version 21.0) [Online Application Software]. Retrieved from https://www.spssau.com, GraphPad Prism 8.0. 2
Change history
26 July 2022
The email address of the corresponding author has been added.
References
Al-Herz W, Bousfiha A, Casanova JL et al (2011) Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2:54
Wang LD, Clark MR (2003) B-cell antigen-receptor signaling in lymphocyte development. Immunology 110(4):411–420
Tsukada S, Saffran DC, Rawlings DJ et al (1993) Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72(2):279–290
Lindvall JM, Blomberg KE, Väliaho J et al (2005) Bruton’s tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev 203:200–215
López-Granados E, Pérez de Diego R, Ferreira Cerdán A et al (2005) A genotype-phenotype correlation study in a group of 54 patients with X-linked agammaglobulinemia. J Allergy Clin Immunol 116(3):690–697
Lederman HM, Winkelstein JA (1985) X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 64:145–156
Hansel TT, Haeney MR, Thompson RA (1987) Primary hypogammaglobulinemia and arthritis. Br Med J (Clin Res Ed) 295:174–175
Winkelstein JA, Marino MC, Lederman HM et al (2006) X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 85:193–202
Lee AH, Levinson AI, Schumacher HR Jr (1993) Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum 22:252–264
Kareva L, Mironska K, Stavric K (2013) Joint disease in children with X-linked agammaglobulinemia. J IMAB 19(3):457–460
Seidel MG, Kindle G, Gathmann B et al (2019) The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract 7(6):1763–1770
Kanegane H, Futatani T, Wang Y et al (2001) (2001) Clinical and mutational characteristics of X-linked agammaglobulinemia and its carrier identified by flow cytometric assessment combined with genetic analysis. J Allergy Clin Immunol 108:1012–1020
Hofer M, Southwood TR (2002) Classification of childhood arthritis. Best Pract Res Clin Rheumatol 16(3):379–396
Malattia C, Damasio MB, Pistorio A et al (2011) Development and preliminary validation of a paediatric-targeted MRI scoring system for the assessment of disease activity and damage in juvenile idiopathic arthritis[J]. Ann Rheum Dis 70(3):440–446
Mourão AF, Santos MJ, Melo-Gomes J et al (2014) Using the Juvenile Arthritis Disease Activity Score based on erythrocyte sedimentation rate or C-reactive protein level: results from the Portuguese register. Arthritis Care Res (Hoboken) 66(4):585–591
Østergaard M, Peterfy CG, Bird P et al (2017) The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: updated recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol 44(11):1706–1712
Moin M, Aghamohammadi A, Farhoudi A et al (2004) X-linked agammaglobulinemia: a survey of 33 Iranian patients. Immunol Invest 33:81–93
Pac M, Bernatowska EA, Kierkuś J et al (2017) Gastrointestinal disorders next to respiratory infections as leading symptoms of X-linked agammaglobulinemia in children-34-year experience of a single center. Arch Med Sci 13:412–417
Singh S, Rawat A, Suri D et al (2016) X-linked agammaglobulinemia: twenty years of single-center experience from North West India. Ann Allergy Asthma Immunol 117:405–411
Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C et al (2014) Autoimmunity and inflammation in X-linked agammaglobulinemia. J Clin Immunol 34(6):627–632
Plebani A, Soresina A, Rondelli R et al (2002) Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol 104(3):221–230
Lee PP, Chen TX, Jiang LP et al (2010) Clinical characteristics and genotype-phenotype correlation in 62 patients with X-linked agammaglobulinemia. J Clin Immunol 30(1):121–131
Nguyen JC, Lee KS, Thapa MM et al (2017) US evaluation of juvenile idiopathic arthritis and osteoarticular infection. Radiographics 37(4):1181–1201
Pascoli L, Wright S, McAllister C et al (2010) Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol 37(11):2409–2414
Spârchez M, Fodor D, Miu N (2010) The role of power Doppler ultrasonography in comparison with biological markers in the evaluation of disease activity in juvenile idiopathic arthritis. Med Ultrason 12(2):97–103
Quartier P, Debré M, De Blic J et al (1999) Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr 134(5):589–596
Lederman HM, Winkelstein JA (1985) X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 64(3):145–156
Franz A, Webster AD, Furr PM et al (1997) Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Br J Rheumatol 36:661–668
Thyss A, el Baze P, Lefebvre JC et al (1990) Dermatomyositis-like syndrome in X-linked hypogammaglobulinemia. Case-report and review of the literature. Acta Derm Venereol 70:309–331
Zhu Z, Kang Y, Lin Z et al (2015) X-linked agammaglobulinemia combined with juvenile idiopathic arthritis and invasive Klebsiella pneumoniae polyarticular septic arthritis. Clin Rheumatol 34(2):397–401
Pessach IM (2010) The relationship of X-linked primary immunodeficiencies and autoimmunity. Curr Allergy Asthma Rep 10(5):311–319
Amaya-Uribe L, Rojas M, Azizi G et al (2019) Primary immunodeficiency and autoimmunity: a comprehensive review. J Autoimmun 99:52–72
Ng YS, Wardemann H, Chelnis J et al (2004) Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med 200(7):927–934
Lee KG, Xu S, Wong ET et al (2008) Bruton’s tyrosine kinase separately regulates NF-kappa B p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. J Biol Chem 283(17):11189–11198
Yong PF, Workman S, Wahid F et al (2008) Selective deficits in blood dendritic cell subsets in common variable immunodeficiency and X-linked agammaglobulinemia but not specific polysaccharide antibody deficiency. Clin Immunol 127(1):34–42
López-Herrera G, Vargas-Hernández A, González-Serrano ME et al (2014) Bruton’s tyrosine kinase–an integral protein of B cell development that also has an essential role in the innate immune system. J Leukoc Biol 95(2):243–250
Liu X, Zhan Z, Li D et al (2011) Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase BTK. Nat Immunol 12(5):416–424
Danks L, Workman S, Webster D et al (2011) Elevated cytokine production restores bone resorption by human Btk-deficient osteoclasts. J Bone Miner Res 26(1):182–192
Schett G (2008) Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity 41(3):224–229
Ringold S, Angeles-Han ST, Beukelman T et al (2019) 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken) 71(6):717–734
Sukumaran S, Marzan K, Shaham B et al (2011) A child with x-linked agammaglobulinemia and enthesitis-related arthritis. Int J Rheumatol 2011:175973
García-García E, Staines-Boone AT, Vargas-Hernández A et al (2016) Clinical and mutational features of X-linked agammaglobulinemia in Mexico. Clin Immunol 165:38–44
Honda F, Kano H, Kanegane H et al (2012) The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol 13(4):369–378
Broides A, Yang W, Conley ME (2006) Genotype/phenotype correlations in X-linked agammaglobulinemia. Clin Immunol 118(2–3):195–200
Acknowledgements
The author would like to thank all the doctors of the Department of Rheumatology and Immunology, Children’s Hospital of Chongqing Medical University for their assistance with obtaining the clinical data and their help in the study design and paper writing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis and the first draft of the manuscript were performed by Ran Qing-qi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Children’s Hospital, Chongqing Medical University (No. 001–2013), and written informed consent was obtained from patients before writing this manuscript.
Consent to participate
Written informed consent was obtained from the patients before writing this manuscript.
Consent for publication
All authors have seen and approved the final version of the manuscript being submitted. This article is the authors’ original work, has not received prior publication, and is not under consideration for publication elsewhere.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qing-qi, R., Ya-wen, L., Huan, C. et al. Retrospective study of 98 patients with X-linked agammaglobulinemia complicated with arthritis. Clin Rheumatol 41, 1889–1897 (2022). https://doi.org/10.1007/s10067-022-06095-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06095-1