Abstract
Objective
T cell-mediated immune response plays a key role in Takayasu arteritis (TAK). Although previous studies have showed the roles of CD4+T cell and its subsets in TAK, the change of CD8+ T cell subsets remains unclear. This study investigated the role of CD8+ T cell subsets in TAK.
Methods
The study consisted of 56 TA patients and 51 healthy controls. The percentages of CD8+T cells, CD8+GranzymeB+ T cells, CD8+Perforin+ T cells, and CD8+IFN-γ+ T cells in blood samples were analyzed by flow cytometry.
Results
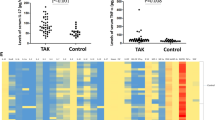
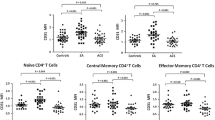
We found that the percentages of CD8+GranzymeB+ T cells (P = 0.030), CD8+Perforin+ T cells (P = 0.000), and CD8+IFN-γ+ T cells (P = 0.002) in CD8+T cells were higher in TAK patients compared to control group. After 6 months of treatment, the proportion of CD8+T cells in lymphocytes were significantly lower in TAK patients than the baseline assessment (P = 0.033). A lower ratio of CD8+GranzymeB+ T cells/CD8+ T cells were showed in TAK patents after treatment compared with TAK patients before treatments (P = 0.011). The change of CD8+GranzymeB+ T cells/CD8+ T cells ratio was positively correlated with the change of ITAS (r = 0.721, P = 0.002) and ITAS-A (r = 0.637, P = 0.008). Finally, the immunofluorescence staining showed the infiltration of CD8+ Granzyme B + cells in the aortic tissue of TAK patients.
Conclusion
Our results disclose that the CD8+ T lymphocytes may play a role in TAK pathogenesis. Targeting CD8+GranzymeB+ T lymphocytes or Granzyme B inhibitors could be a potential therapeutic approach for the treatment of TAK.
Key Points • Our study investigated role the of CD8+ T cell subsets in TAK. • We found the percentages of CD8+GranzymeB+ T cells, CD8+Perforin+ T cells, and CD8+IFN-γ+ T cells in CD3+CD8+T cells were higher in TAK patients. • The proportion of CD8+T cells in lymphocytes and the ratio of CD8+GranzymeB+ T cells/CD8+ T cells were significantly lower in TAK patients after treatment. |



Similar content being viewed by others
Data and material availability
No additional data available.
Code availability
Not applicable.
References
Zaldivar Villon MLF, de la Rocha JAL, Espinoza LR (2019) Takayasu arteritis: recent developments. Curr Rheumatol Rep 21(9):45. https://doi.org/10.1007/s11926-019-0848-3
Tombetti E, Mason JC (2019) Takayasu arteritis: advanced understanding is leading to new horizons. Rheumatology (Oxford) 58(2):206–219. https://doi.org/10.1093/rheumatology/key040
Weyand CM, Goronzy JJ (2003) Medium- and large-vessel vasculitis. N Engl J Med 349(2):160–169. https://doi.org/10.1056/NEJMra022694
Kermani TA (2019) Takayasu arteritis and giant cell arteritis: are they a spectrum of the same disease? Int J Rheum Dis 22(Suppl 1):41–48. https://doi.org/10.1111/1756-185X.13288
de Souza AW, de Carvalho JF (2014) Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 48–49:79–83. https://doi.org/10.1016/j.jaut.2014.01.012
Watanabe Y, Miyata T, Tanemoto K (2015) Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 132(18):1701–1709. https://doi.org/10.1161/CIRCULATIONAHA.114.012547
Espinoza JL, Ai S, Matsumura I (2018) New insights on the pathogenesis of Takayasu arteritis: revisiting the microbial theory. Pathogens 7 (3). https://doi.org/10.3390/pathogens7030073
Li T, Gao N, Cui W, Zhao L, Pan L (2020) Natural killer cells and their function in Takayasu’s arteritis. Clin Exp Rheumatol 38(Suppl 124 (2)):84–90
Keser G, Aksu K, Direskeneli H (2018) Discrepancies between vascular and systemic inflammation in large vessel vasculitis: an important problem revisited. Rheumatology (Oxford) 57(5):784–790. https://doi.org/10.1093/rheumatology/kex333
Weyand CM, Goronzy JJ (2013) Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 9(12):731–740. https://doi.org/10.1038/nrrheum.2013.161
Palmer E (2003) Negative selection–clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol 3(5):383–391. https://doi.org/10.1038/nri1085
Caza T, Landas S (2015) Functional and phenotypic plasticity of CD4(+) T cell subsets. Biomed Res Int 2015:521957. https://doi.org/10.1155/2015/521957
Samson M, Corbera-Bellalta M, Audia S, Planas-Rigol E, Martin L, Cid MC, Bonnotte B (2017) Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun Rev 16(8):833–844. https://doi.org/10.1016/j.autrev.2017.05.014
Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, Terrier B, Geri G, Rosenzwajg M, Klatzmann D, Fourret P, Cluzel P, Chiche L, Gaudric J, Koskas F, Cacoub P (2015) Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 67(5):1353–1360. https://doi.org/10.1002/art.39037
Gao N, Cui W, Zhao LM, Li TT, Zhang JH, Pan LL (2020) Contribution of Th2-like Treg cells to the pathogenesis of Takayasu’s arteritis. Clin Exp Rheumatol 38 Suppl 124(2):48–54
Pan LL, Du J, Gao N, Liao H, Wan J, Ci WP, Yang C, Wang T (2016) IL-9-producing Th9 cells may participate in pathogenesis of Takayasu’s arteritis. Clin Rheumatol 35(12):3031–3036. https://doi.org/10.1007/s10067-016-3399-2
Uppal SS, Verma S (2003) Analysis of the clinical profile, autoimmune phenomena and T cell subsets (CD4 and CD8) in Takayasu’s arteritis: a hospital-based study. Clin Exp Rheumatol 21(6 Suppl 32):S112-116
Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS (1994) Takayasu arteritis. Ann Intern Med 120(11):919–929. https://doi.org/10.7326/0003-4819-120-11-199406010-00004
Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM, Jeyaseelan L, Lawrence A, Bacon PA, Vasculitis IR, g, (2013) Development and initial validation of the Indian Takayasu clinical activity score (ITAS2010). Rheumatology (Oxford) 52(10):1795–1801. https://doi.org/10.1093/rheumatology/ket128
Lee H, Sun Y, Patti-Diaz L, Hedrick M, Ehrhardt AG (2019) High-throughput analysis of clinical flow cytometry data by automated gating. Bioinform Biol Insights 13:1177932219838851. https://doi.org/10.1177/1177932219838851
Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, Khalidi NA, Langford CA, Monach PA, Seo P, Warrington KJ, Ytterberg SR, Hoffman GS, Merkel PA (2012) Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis 71(8):1329–1334. https://doi.org/10.1136/annrheumdis-2011-200795
Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z (2011) Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev 11(1):61–67. https://doi.org/10.1016/j.autrev.2011.08.001
Dos Santos JP, Artigiani Neto R, Mangueira CLP, Filippi RZ, Gutierrez PS, Westra J, Brouwer E, de Souza AWS (2020) Associations between clinical features and therapy with macrophage subpopulations and T cells in inflammatory lesions in the aorta from patients with Takayasu arteritis. Clin Exp Immunol 202(3):384–393. https://doi.org/10.1111/cei.13489
Nityanand S, Giscombe R, Srivastava S, Hjelmstrom P, Sanjeevi CB, Sinha N, Grunewald J, Lefvert AK (1997) A bias in the alphabeta T cell receptor variable region gene usage in Takayasu’s arteritis. Clin Exp Immunol 107(2):261–268. https://doi.org/10.1111/j.1365-2249.1997.295-ce1186.x
Matsumoto K, Suzuki K, Yoshimoto K, Seki N, Tsujimoto H, Chiba K, Takeuchi T (2019) Significant association between clinical characteristics and changes in peripheral immuno-phenotype in large vessel vasculitis. Arthritis Res Ther 21(1):304. https://doi.org/10.1186/s13075-019-2068-7
Gravano DM, Hoyer KK (2013) Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun 45:68–79. https://doi.org/10.1016/j.jaut.2013.06.004
Samson M, Ly KH, Tournier B, Janikashvili N, Trad M, Ciudad M, Gautheron A, Devilliers H, Quipourt V, Maurier F, Meaux-Ruault N, Magy-Bertrand N, Manckoundia P, Ornetti P, Maillefert JF, Besancenot JF, Ferrand C, Mesturoux L, Labrousse F, Fauchais AL, Saas P, Martin L, Audia S, Bonnotte B (2016) Involvement and prognosis value of CD8(+) T cells in giant cell arteritis. J Autoimmun 72:73–83. https://doi.org/10.1016/j.jaut.2016.05.008
Boita M, Guida G, Circosta P, Elia AR, Stella S, Heffler E, Badiu I, Martorana D, Mariani S, Rolla G, Cignetti A (2014) The molecular and functional characterization of clonally expanded CD8+ TCR BV T cells in eosinophilic granulomatosis with polyangiitis (EGPA). Clin Immunol 152(1–2):152–163. https://doi.org/10.1016/j.clim.2014.03.001
Kurata A, Saito A, Hashimoto H, Fujita K, Ohno SI, Kamma H, Nagao T, Kobayashi S, Yamashina A, Kuroda M (2019) Difference in immunohistochemical characteristics between Takayasu arteritis and giant cell arteritis: it may be better to distinguish them in the same age. Mod Rheumatol:1–10. https://doi.org/10.1080/14397595.2019.1570999
de Araujo-Souza PS, Hanschke SC, Viola JP (2015) Epigenetic control of interferon-gamma expression in CD8 T cells. J Immunol Res 2015:849573. https://doi.org/10.1155/2015/849573
Bertoletti A, Ferrari C (2016) Adaptive immunity in HBV infection. J Hepatol 64(1 Suppl):S71–S83. https://doi.org/10.1016/j.jhep.2016.01.026
Deng Q, Luo Y, Chang C, Wu H, Ding Y, Xiao R (2019) The emerging epigenetic role of cd8+t cells in autoimmune diseases: a systematic review. Front Immunol 10:856. https://doi.org/10.3389/fimmu.2019.00856
Prakash MD, Munoz MA, Jain R, Tong PL, Koskinen A, Regner M, Kleifeld O, Ho B, Olson M, Turner SJ, Mrass P, Weninger W, Bird PI (2014) Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity 41(6):960–972. https://doi.org/10.1016/j.immuni.2014.11.012
Bovenschen N, Kummer JA (2010) Orphan granzymes find a home. Immunol Rev 235(1):117–127. https://doi.org/10.1111/j.0105-2896.2010.00889.x
Boivin WA, Cooper DM, Hiebert PR, Granville DJ (2009) Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest 89(11):1195–1220. https://doi.org/10.1038/labinvest.2009.91
Mollah ZU, Graham KL, Krishnamurthy B, Trivedi P, Brodnicki TC, Trapani JA, Kay TW, Thomas HE (2012) Granzyme B is dispensable in the development of diabetes in non-obese diabetic mice. PLoS ONE 7(7):e40357. https://doi.org/10.1371/journal.pone.0040357
Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF (2005) Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 52(1):201–211. https://doi.org/10.1002/art.20745
Parkinson LG, Toro A, Zhao H, Brown K, Tebbutt SJ, Granville DJ (2015) Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell 14(1):67–77. https://doi.org/10.1111/acel.12298
Saito Y, Kondo H, Hojo Y (2011) Granzyme B as a novel factor involved in cardiovascular diseases. J Cardiol 57(2):141–147. https://doi.org/10.1016/j.jjcc.2010.10.001
Annunziato F, Romagnani C, Romagnani S (2015) The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135(3):626–635. https://doi.org/10.1016/j.jaci.2014.11.001
Schoenborn JR, Wilson CB (2007) Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 96:41–101. https://doi.org/10.1016/S0065-2776(07)96002-2
Tripathy NK, Chauhan SK, Nityanand S (2004) Cytokine mRNA repertoire of peripheral blood mononuclear cells in Takayasu’s arteritis. Clin Exp Immunol 138(2):369–374. https://doi.org/10.1111/j.1365-2249.2004.02613.x
Savioli B, Abdulahad WH, Brouwer E, Kallenberg CGM, de Souza AWS (2017) Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev 16(10):1071–1078. https://doi.org/10.1016/j.autrev.2017.07.023
Bhat P, Leggatt G, Waterhouse N, Frazer IH (2017) Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis 8(6):e2836. https://doi.org/10.1038/cddis.2017.67
Regnier P, Le Joncour A, Maciejewski-Duval A, Desbois AC, Comarmond C, Rosenzwajg M, Klatzmann D, Cacoub P, Saadoun D (2020) Targeting JAK/STAT pathway in Takayasu’s arteritis. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2019-216900
Funding
The present study was supported by grants from National Natural Science Foundation of China (91739111, 81900448).
Author information
Authors and Affiliations
Contributions
Lili Pan contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Taotao Li, Na Gao, Juan Du, and Xuemei Shi. Junming Zhu, Zhiyu Qiao and Shichao Guo collected the samples. Wei Cui and Limin Zhao performed the experiments. The first draft of the manuscript was written by Taotao Li. Lili Pan is the corresponding author of the manuscript who guided the writing and made the corrections of the whole paper. All authors declare no potential conflicts of interest.
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital (approval no. 2019067X).
Consent to participation
All subjects provided written informed consent. The privacy rights of human subjects are always be observed.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, T., Gao, N., Cui, W. et al. The role of CD8+ Granzyme B+ T cells in the pathogenesis of Takayasu’s arteritis. Clin Rheumatol 41, 167–176 (2022). https://doi.org/10.1007/s10067-021-05903-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05903-4