Abstract
The majority of the medical fraternity is continuously involved in finding new therapeutic schemes, including antimalarial medications (AMDs), which can be useful in combating the 2019-nCoV: coronavirus disease (COVID-19). For many decades, AMDs have been widely used in the treatment of malaria and various other anti-inflammatory diseases, particularly to treat autoimmune disorders of the connective tissue. The review comprises in vitro and in vivo studies, original studies, clinical trials, and consensus reports for the analysis, which were available in medical databases (e.g., PubMed). This manuscript summarizes the current knowledge about chloroquine (CQ)/hydroxychloroquine (HCQ) and shows the difference between their use, activity, recommendation, doses, and adverse effects on two groups of patients: those with rheumatic and viral diseases (including COVID-19). In the case of connective tissue disorders, AMDs are prescribed for a prolonged duration in small doses, and their effect is observed after few weeks, whereas in the case of viral infections, they are prescribed in larger doses for a short duration to achieve a quick saturation effect. In rheumatic diseases, AMDs are well tolerated, and their side effects are rare. However, in some viral diseases, the effect of AMDs is questionable or not so noticeable as suggested during the initial prognosis. They are mainly used as an additive therapy to antiviral drugs, but recent studies have shown that AMDs can diminish the efficacy of some antiviral drugs and may cause respiratory, kidney, liver, and cardiac complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimalarial drugs (AMDs) have been used in the treatment of malaria and other inflammatory diseases for over 70 years. Chloroquine (CQ) was first synthesized AMD in Germany by Bayer in 1934. It was used as a synthetic replacement for natural quinine in the treatment of malaria [1]. However, due to drug resistance, CQ is less often implemented in the treatment of malaria.
Over the last decades, the anti-inflammatory and immunomodulatory properties of AMDs became the main reason for their use in rheumatic diseases [2]. They are recommended in the treatment of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), sarcoidosis, dermatomyositis, Sjögren’s syndrome, chronic juvenile arthritis, psoriatic arthritis, and various other autoimmune diseases, because of their good efficacy and acceptable safety profiles [3]. AMDs are advantageous because of their excellent safety profile (especially hydroxychloroquine (HCQ)). These medications reduce skin lesions exacerbated by ultraviolet light, arthralgia, and myalgia, improve fatigue, decrease disease activity, and diminish cardiovascular risk [4,5,6,7,8]. They have immunomodulatory, antiviral, antitumor, and antithrombotic effects and play a beneficial role in the regulation of metabolism [3].
Recently, AMDs have been used to treat many other viral diseases. The earlier studies of AMDs as antiviral agents were not very encouraging (e.g., in Chikungunya virus) [9], and the early reports indicated that AMDs had no effect on viral diseases in humans [10, 11]. More recent data show some efficacy of AMDs in HIV and novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-1). However, initially, the treatment of 2019-nCoV: coronavirus disease (COVID-19) with AMDs was met with skepticism, and they were not recommended in quickly spreading viral infections [12]. Nevertheless, chloroquine (CQ) and HCQ were approved for the treatment of COVID-19 based on their in vitro antiviral activity [13]. The decision of using AMDs in the treatment of COVID-19 was also supported by the anti-inflammatory activity and inhibition of the production of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6. This effect is named a “cytokine storm” and is reduced by AMDs [14, 15]. Subsequent studies and clinical experience have underlined the efficacy and the beneficial effects of AMDs in patients with COVID-19, which is primarily based on observational studies in small groups [16, 17]. Later analysis has shown the adverse effect of AMDs in COVID-19 treatment, which caused an increased risk of cardiovascular complications, and even death in severely infected patients [18, 19].
Given the fact that AMDs are extensively used in rheumatic and viral diseases, in this review, we discuss the mechanism, indications, recommended or suggested doses, efficiency, and adverse effects of AMDs. We also present a critical point of view and the latest evidence of the beneficial or harmful effects of AMDs in various clinical disorders, underling their impact on SARS-CoV-2 infection.
AMDs—properties and mechanism of action in rheumatic diseases
AMDs are lipophilic drugs and have weak alkaline properties. They inhibit lysosomal degradation/autophagy either by altering lysosomal acidification or by inhibiting the levels of lysosomal-associated membrane proteins (LAMPs) [20]. AMDs encompass three drugs: CQ, HCQ, and quinacrine; however, CQ and HCQ are most often used in rheumatic and viral diseases [21]. HCQ differs from CQ in that it contains a hydroxyl group at the end of the side chain. Both CQ and HCQ show similar pharmacokinetic behavior and are quickly absorbed by the intestine and excreted by the kidneys. Moreover, their recommended doses in rheumatic diseases are well tolerated and have less toxicity [15].
AMDs are particularly useful in skin conditions exacerbated by light (hypersensitivity associated with ultraviolet light). However, the pleiotropic action of AMDs shows a broad spectrum of activity on coagulation and the levels of serum lipid, and glucose resulting in a decreased risk of cardiovascular diseases (CVDs) [5, 17]. Compared with other chronically used immunosuppressants (e.g., methotrexate (MTX), leflunomide (LF), or cyclophosphamide), the use of CQ and HCQ is associated with a reduced risk of infection [22]. Therefore, AMDs are commonly proposed for patients with rheumatic diseases who have mainly viral and bacterial infections and who have comorbidities (Table 1).
AMDs in SLE
SLE is a systemic autoimmune disease, which is characterized by the inflammation of microvasculature leading to the involvement of multiple organs (causing lesions of the skin and mucus membrane, arthritis, neurologic disorder, kidney disease, and hematologic changes) [52]. The production of various auto-antibodies is a significantly important process in the pathogenesis of SLE, from which anti-dsDNA antibody and anti-Sm antibody are essential biomarkers [53]. The primary treatment and management of SLE include glucocorticoids (GCS) and HCQ, as well as nonsteroidal anti-inflammatory drugs, immunosuppressive agents, and biologic therapy [54].
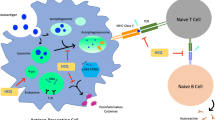
Previous studies have confirmed the efficacy of AMDs in SLE. AMDs demonstrate anti-inflammatory activity (inhibit the synthesis of proinflammatory cytokines) and antioxidant properties (Fig. 1). Recent data show a prominent role of CQ in the treatment of SLE. Along with glucocorticosteroids, CQ is recommended as the first-line treatment for SLE [55]. It alters the pH of lysosomes, where toll-like receptors (TLR)-7 and TLR9 are located. Thereby, it reduces the binding affinity of ds-DNA immune complexes to TLR9 and downregulates the production of interferon in SLE [24].
AMDs show good efficacy mainly in skin diseases, which are associated with joint and muscle inflammation [4]. However, CQ and HCQ are also used in more severe forms of SLE. AMDs decrease the activity of SLE by more than 50%, which helps to lower the doses of GCS [4]. Moreover, they protect against irreversible damage of organs, such as kidney and nervous system, polyserositis and cytopenia, thrombosis development, and loss in bone mass [4, 25, 56]. In patients with SLE associated with antiphospholipid syndrome (APS), which causes severe prothrombotic complications, AMDs decrease the risk of venous thromboembolism (by inhibiting the platelet aggregation and activating the antiphospholipid antibodies) [26]. Thus, they improve patient survival by reducing disease activity and preventing irreversible multiorgan injury [56].
Preclinical studies have shown that HCQ delays the onset of SLE by about 1 year. Patients treated with HCQ had a lower rate of autoantibody accumulation and a decreased number of autoantibody specificities after diagnosis. AMDs postpone the development of flare in the course of SLE [4, 27]. Moreover, a landmark trial showed that discontinuation of HCQ led to a nearly threefold higher risk of lupus exacerbation [57]. It was reported that a low level of serum HCQ in patients with SLE is associated with high disease activity and is considered a strong predictor of flare [58]. Thus, the use of AMDs is characterized by an increase in the long-term survival of patients with SLE [4]. From our clinical practice, AMDs should be continued, both in remission and in a flare.
The beneficial effect of AMDs is based on their reduction of cardiovascular risk factors, such as hyperlipidemia and diabetes mellitus. These medications have a positive influence on insulin resistance among patients with SLE [5]. In pregnancy, AMDs, mainly HCQ, decrease lupus activity and do not have a harmful effect on the fetus [4]. Moreover, they lower the risk of complications during pregnancy [29].
AMDs in RA
RA is characterized by symmetrical inflammatory polyarthritis, often complicated by extra-articular manifestations and an increased rate of morbidity and mortality from CVDs [2, 59]. It affects ~ 0.5 to 1% of the overall population, and it damages cartilage and bone tissue leading to disability and reduced quality of life of the patient [2, 59, 60]. Early treatment of RA is associated with improved outcomes [61]. Immunosuppressive agents, such as MTX and LF, are frequently used in RA; nevertheless, AMDs, sulfasalazine, cyclosporine, and biologic agents are also implemented during the treatment of RA, usually as additive therapy.
AMDs are useful in the treatment of connective tissue disorders that are characterized by palindromic rheumatism [62, 63]. CQ and HCQ also inhibit lysosomal antigen degradation and prevent the activation of autoreactive T cells and subsequent inflammatory responses [30]. HCQ in RA decreases the number of tenders, swollen, and painful joints [33]. But it did not inhibit the radiographic progression [64]. Therefore, they are not used as first-line medications in RA because they do not reduce structural damage sufficiently, especially in comparison with other disease-modifying antirheumatic drugs (DMARDs) [65]. Compared to MTX or LF, AMDs show less activity, and they require a long time to show beneficial clinical improvement. Nevertheless, AMDs can combine with rheumatoid factors. Therefore, they are frequently used as a part of combination therapy [66], or in patients who have very mild disease, or in patients who show contraindications to other medications, such as MTX and LF [33].
AMDs in primary Sjögren’s syndrome (pSS)
AMDs are also used in the treatment of pSS, which is an autoimmune exocrinopathic disorder characterized by lymphocytic infiltration of exocrine glands (mainly salivary and lachrymal glands) [67]. Such lymphoid infiltrations lead to dryness of the eyes and the mouth, as well as other surfaces connected to exocrine glands [68]. In addition to glandular involvement, systemic pathology has also been reported, such as respiratory or neurological disorders [67, 68]. It is noteworthy that pSS is associated with an increased risk of cancer, mainly non-Hodgkin lymphoma [69]. The mainstay of the treatment for the sicca symptoms is local therapy (saliva substitutes and artificial tears) and that for the milder systemic symptoms is HCQ [7]. The treatment of systemic symptoms (extra-glandular manifestations) includes GCS, AMDs, immunosuppressive drugs, and biologic agents [70].
Various studies have shown that HCQ is useful in the treatment of pSS, and it alleviates the signs and symptoms of dry eyes [35, 36]. AMDs show the best effect in the initial stage of the disease without any severe damage to the organ (“burned out” phase), and they improve the course of the illness (decrease fatigue, joint pain, erythrocyte sedimentation rate (ESR), rheumatoid factor (RF) level, gamma globulin level and diminish glandular involvement) [37]. Moreover, they inhibit salivary and serum B-cell activator factor (BAFF) [35, 36]. This helps to reduce disease activity and increase salivary flow [35].
However, some studies do not confirm the efficacy of HCQ in pSS. A randomized controlled trial showed that after 24 weeks of treatment with HCQ, there was no significant improvement in the symptoms of pSS compared with placebo [38]. There was no apparent clinical benefit in the case of dry eyes and systemic inflammation in pSS when HCQ was administered at a dosage of 300 mg daily for 12 weeks [39]. Nevertheless, AMDs have an anti-inflammatory, immunosuppressive, and immunomodulatory role, and they are recommended as first-line treatment for inflammatory musculoskeletal pain associated with pSS (recommendation according to Sjögren’s Syndrome Foundation Clinical Practice Guidelines) [34]. In clinical practice, they are mainly used to treat mild disease without the involvement of organs (e.g., lung fibrosis or severe neuropathy). If an organ is involved, then immunosuppressive medications are required (e.g., cyclophosphamide in pulmonary fibrosis) [34].
AMDs in polymyositis (PM) and dermatomyositis (DM)
Idiopathic inflammatory myopathies (IIM) encompass a heterogeneous group of connective tissue disorders that are characterized by chronic inflammation of striated muscle. The symmetrical weakness of proximal muscle groups, decreased muscle endurance, and chronic inflammation in muscle tissue are the predominant symptoms of IIM [71]. The most common subsets of IIM include adult PM, adult and juvenile DM, immune-mediated necrotizing myopathy, and sporadic inclusion body myositis (IBM) [72]. PM is characterized by symmetrical proximal and progressive weakness in the muscles of limbs, whereas in DM, muscular disorders co-exist with skin abnormalities, e.g., heliotrope rash and Gottron’s papules [73]. Typical complaints include problems in walking, climbing stairs, or lifting an object above their head. Muscle weakness is associated with elevated blood levels of muscle enzymes, particularly creatine phosphokinase (CK). Both PM and DM are often related to nonmuscular manifestations such as interstitial lung disease (ILD), arthropathy, cardiomyopathy, and malignancies [74]. The diagnosis requires testing of auto-antibodies, histological evaluation of a skeletal muscle biopsy, and further tests, including muscle magnetic resonance imaging (MRI) and electromyography (EMG) [75]. The treatment of PM/DM includes GCS and immunosuppressants. AMDs are also used in the treatment of inflammatory myopathies, particularly the cutaneous symptoms of DM [49, 76].
Various case reports or clinical case series have shown beneficial effects of AMDs. According to these reports, AMDs can be administered alone or in combination with GCS [49,50,51]. HCQ is usually used in combination with GCS and is good to treat skin conditions of patients with DM. It can completely resolve the lesions on the skin and may enable GCS tapering [50]. The efficacy of AMDs has been demonstrated in adults [50] and childhood DM [51]. Some studies highlight the positive effect of AMDs in the treatment of cutaneous lesions in dermatomyositis [50]. However, they may also cause mild-to-moderately severe vacuolar myopathy and may be responsible for severe and death-leading cases with ventilatory failure [77]. Moreover, an increased risk of Herpes zoster infection has been described in patients with PM/DM [78]. In addition to these casuistic descriptions, AMDs have shown excellent safety profiles, but their use in inflammatory myopathies should be carefully monitored in clinical practice, which can be achieved by analyzing patient symptoms and measurement of muscle enzymes [3, 15, 79].
AMDs used in sarcoidosis
Sarcoidosis is a multisystem granulomatous disease characterized by the presence of noncaseating granulomas [80]. The course of this disease can be self-remitting within 12 to 36 months (in more than 60% of the patients) or can become chronic, requiring prolonged treatment (in approximately 10% to 30% of the patients) [81]. In the case of patients in whom sarcoidosis does not cause any significant symptoms or organ dysfunction, the treatment is not compulsory. Systemic therapy is required in life-threatening conditions with organ involvement such as advanced pulmonary fibrosis or pulmonary hypertension, central nervous system disorders, heart, or renal sarcoidosis [82]. If organ damage is observed, the first-line treatment for sarcoidosis is GCS, which reveals therapeutic effects. In rare cases, the disease fails to respond to GCS monotherapy or combined GCS and second-line immunosuppressants. Combined therapy is mainly used to achieve disease control or GCS sparing, mostly in cases, when multiple side effects are present [43, 82].
When treatment is indicated, oral corticosteroids are usually recommended because they show good efficacy in a short period. AMDs are recommended mainly in cutaneous sarcoidosis, and they cause the regression of granulomatous changes on the skin (the minimum doses of CQ and HCQ have the same effect as the maximum doses) [42]. AMDs are suspected to impair the release of cytokines and weaken the process of antigen presentation by monocytes, macrophages, and dendritic cells to CD4-positive helper T cells [40]. Moreover, CQ has demonstrated a positive effect in the treatment of symptomatic persistent pulmonary sarcoidosis, but the data are quite limited [43]. In sarcoidosis, AMDs are used in patients who fail to respond to GCS treatment or show contraindication to GCS. Since sarcoidosis is often a chronic condition, long-term treatment with GCS may cause significant toxicity. Therefore, GCS-sparing agents are often indicated in patients requiring prolonged therapy [82]. Therefore, AMDs are mainly recommended in cutaneous forms of sarcoidosis, usually along with rapidly working GCS, whose doses could be slowly decreased.
Mechanism of antiviral action of AMDs
AMDs demonstrate broad-spectrum antiviral effects (Table 2). For example, more than 20 years ago, CQ was shown to inhibit HIV-dependent replication [83]. Other examples include inhibition of RNA viruses such as hepatitis A or C; influenza A, B, and H5N1 viruses A; poliovirus; rabies; dengue; or Ebola, as well as DNA viruses (hepatitis B and herpes simplex virus) [84]. Moreover, the positive effect of AMDs has been reported in the treatment of different types of coronaviruses such as CoV-229E (in vitro studies) [85], HCoV-OC43 (studies on animal models) [86, 87], and SARS-CoV-1 [11, 88].
Initially, the suspected mechanism of action of CQ against SARS-CoV-2 was based on its activity on other viruses, mainly SARS-CoV-1, dengue-2 virus, and influenza A H5N1 virus [11, 15, 49, 94]. In viral infections, AMDs can impair the replication of some viruses by reducing the efficiency of endosome-mediated virus entry [15, 94]. These drugs also decrease the activity of low-pH–dependent proteases in trans-Golgi vesicles [15]. It was shown that CQ inhibits the initial step of the viral cycle by interfering with the binding of viral particles to the cell surface receptor. It inhibits quinone reductase 2, which participates in the biosynthesis of sialic acids [104], which are part of the transmembrane proteins and are involved in the recognition of ligands. Many coronaviruses (e.g., HCoV-O43) use sialic acid residues as receptors [86]. Thus, these data gave the background to suspect that AMDs might be active in SARS-CoV-2 infection.
AMDs in SARS-CoV-2 infection
Disease caused by the new beta-coronavirus SARS-CoV-2 was first reported in the Chinese city of Wuhan, but the rapidly spreading infection with life-threatening complications caused the WHO to declare a global pandemic. This virus is similar in structure to the SARS-CoV-1, and it contains 80% of the same nucleotides. The disease caused by SARS-CoV-2 is called COVID-19 (coronavirus disease 2019) [105]. Since the epidemic started, the entire medical world began to look for new and effective drugs, which will help to control the spread of the disease. Because CQ and HCQ have been reported to have broad-spectrum antiviral activity, they have been considered for use in the treatment of COVID-19 [106].
The exact antiviral mechanisms of AMDs are not known, but many mechanisms of action have been suggested (Fig. 2). The effect of these drugs in inhibiting SARS-CoV-2 is based on the fact that CQ influences both entry and intracellular stages of the SARS-CoV-2 replication in Vero E6 cells [107]. Many coronaviruses bind to sialic acid and this process is required for viral replication. It has been proved that CQ decreases the biosynthesis of sialic acid. Therefore, it is suspected that SARS-CoV-2 similarly binds to sialic acid, and this binding can be inhibited by CQ [108].
Another mechanism, which is involved in the activity of CQ is its interaction with the surface receptor of the angiotensin-2 converting enzyme (ACE2). S protein of the virus attaches to host receptor ACE2. Similar to SARS-CoV-1, SARS-CoV-2 virus also uses the same ACE2 receptor. These receptors are present on the surface of the lung, heart, kidneys, and intestines [96, 109]. Recent data shows that SARS-CoV-2 binds to ACE2 expressed on pneumocytes [109]. CQ reduces the ACE2 receptor terminal glycosylation on the surface of cells and interferes with the binding of SARS-CoV-2 to the ACE2 receptor [97, 110].
The penetration of coronavirus also occurs through the endosomal pathway [111]. AMDs increase the endosomal pH of the cell, which affects the binding of the virus to the cell and interferes with the glycosylation of the cell surface receptors [15, 94]. An increase in the endosomal pH by CQ inhibits the formation of cathepsins, which require an acidic environment for the optimal cleavage of SARS-CoV-2 spike protein [112]. Thus, CQ, by modulating the acidification of endosomes, prevents virus/cell fusion with host cells and subsequent viral replication [97, 107]. In in vitro studies, CQ inhibits the replication of SARS-CoV-2 at a low-micromolar concentration (EC50 = 1.13 μM in Vero E6 cells) [107].
An additional mechanism of action of CQ, which is suspected in COVID-19, includes the inhibition of MAPK kinases, inhibition of proteolysis of the M protein and alteration of virion assembly, and budding. CQ might indirectly decrease the production of proinflammatory mediators and/or by activating anti-SARS-CoV-2 CD8+ T cells and the inhibition of production of cytokines such as IL-1, IL-6, and TNF-α [113]. The inhibition of the production of proinflammatory mediators may reduce the effects of a “cytokine storm.” Excess cytokine synthesis results from the Th-2-dependent response during the course of coronavirus infection, which leads to the development of various symptoms [14, 15]. Thus, decreasing the excess production of proinflammatory markers can deteriorate the severity of the viral infection. Moreover, during the initial phase of SARS-CoV-2 infection, AMDs increase the secretion of Th-2 helper lymphocyte cytokines (IL-4 and IL-10) [114]. In addition, CQ prevents exacerbated activation of Th-1 lymphocytes, which are responsible for the development of acute respiratory distress syndrome (ARDS) [15].
In addition to inhibiting the entry of viruses, CQ inhibits the secretion of IL-2, which is involved in the differentiation of T-2 cells into the Th-2 subset [115]. As a result, the proliferation of Th-2 lymphocytes is reduced. Thus, it is hypothesized that CQ and HCQ impair the immune response to SARS-CoV-2 infection and prevent the progressive course of the disease [114]. In summary, AMDs in coronavirus infection interfere with ACE2 to block the invasion of the virus, increase endosomal pH required for the fusion of the virus, and show mild immune suppression.
AMDs in clinical practice
On 13 March 2020, the Office of Registration of Medicinal Products, Medical Devices, and Biocidal Products in Warsaw (Poland) issued a new therapeutic indication for medicines containing CQ, consisting of the addition of “supportive treatment in beta-coronavirus infections such as SARS-CoV-1, MERS-CoV, and SARS-CoV-2.” This drug was used to treat patients from the Wuhan region and several other cities in China. Initially, CQ and HCQ were approved for the treatment of COVID-19 based on their in vitro antiviral activity [13] and antiviral properties against other viral infections, e.g., HIV [83] and SARS-CoV-1 [97, 116]. Given these facts, the first clinical trials tried to check an inhibitory effect of AMDs on viremia [15, 16]. In rheumatic diseases, AMDs reveal their full anti-inflammatory and immune regulatory activity within a few weeks (about 1–3 months), but their antiviral effect is quite rapid [17].
The preliminary observation revealed that CQ inhibits the progression of clinical and radiological signs of pneumonia, facilitates the elimination of the virus up to the stage of indeterminate viremia, shortens the duration of the disease, reduces hospitalization time, and has no severe side effects [16]. HCQ used in COVID-19 for 3 days enhanced virus clearance, and azithromycin reinforced the antiviral efficacy [17].
CQ versus HCQ
Because CQ and HCQ (a derivative of chloroquine) have similar chemical structures and mechanisms of action, they are equally used in the treatment of viral infection. Both AMDs are active against SARS-CoV-2 under in vitro studies; however, HCQ seems to differ from CQ in a few aspects [13, 107]. In vitro [13] and in vivo study has confirmed [98] the effect of HCQ in COVID-19 infection. But HCQ was more potent than CQ in inhibiting SARS-CoV-2 infection. The therapeutic effect of HCQ was confirmed by a randomized controlled clinical trial (ChiCTR2000029559). This study revealed that patients on HCQ therapy had significantly shortened clinical recovery time, quick resolution of symptoms (such as fever and cough), and more than 80% of patients had improved pneumonia compared to the control group without HCQ therapy [98]. HCQ demonstrated substantially fewer side effects [117] and seems to show a more potent antiviral activity than that of CQ [13, 107]. Therefore, HCQ was recommended in SARS-CoV-2 treatment in an expert consensus statement from Shanghai [118]. Moreover, it does not cause multiple drug–drug interactions than that of CQ [13].
Based on an in vitro study [107], HCQ was rapidly introduced into clinical use, and preliminary reports suggested an improved viral clearance and clinical outcomes in patients with COVID-19 receiving a 10-day course of HCQ [16]. A small French pilot study, randomizing 36 patients with COVID-19, suggested accelerated viral clearance in patients treated with a combination of HCQ and azithromycin [17]. Moreover, HCQ equally as CQ influences macrophage activation and cytokine storm [119]. This results in the decreased production of proinflammatory mediators such as IL-1, IL-6, and prostaglandins [120]. Moreover, HCQ has antithrombotic effects, which prevents the formation of micro-thrombus during endothelial damage caused by SARS-CoV-2 infection [121, 122].
The earliest use of AMDs has shown that HCQ shows a positive effect in the treatment of COVID-19 when given in combination with azithromycin leading to a complete and rapid viral clearance (clinical trials) [17]. However, some studies did not confirm this hypothesis. Magagnoli et al. conducted a retrospective study and analyzed the use of HCQ with and without azithromycin and with the control group (without HCQ) [102]. They observed that the risk of death from any cause was higher in the HCQ group (adjusted HR = 2.61; 95% CI: 1.10–6.17; p = 0.03) than that of the non-HCQ group (the authors analyzed a cohort of 368 males, predominantly African American veterans). Moreover, the risk of necessary ventilation or the risk of death after ventilation was also not significantly different among the three groups [102]. Similar doses of HCQ and azithromycin were used in the prospective case series on 11 patients with severe infection with SARS-CoV-2, in which viral clearance and clinical outcome were not improved by this combination. Molina et al. [123] conducted a study on 181 patients with severe infection with SARS-CoV-2 showed that HCQ did not significantly reduce ICU admission, death on the seventh day after hospitalization, or reduce the incidence of ARDS compared to those who did not receive HCQ [124].
The dose of CQ and HCQ in COVID-19 treatment
In vitro study confirmed the inhibitory effect of CQ against SARS-CoV-2 at concentrations that is much lower than cytotoxic level [107]. An excellent safety profile and antiviral activity allowed to consider CQ in the treatment of COVID-19. In a study conducted on 13 severe to critically ill patients with COVID-19 admitted in the ICU, the authors recommended the use of HCQ 800 mg once on the first day to rapidly achieve therapeutic levels, followed by 200 mg twice daily for 7 days [125]. A similar dosage was recommended by Yao et al. for HCQ; they administered 400 mg bid on the first day and the maintenance dose of 200 mg bid for 4 days [13]. The guidelines of SIMIT Lombardy section included CQ/HCQ in the treatment of severe to critically ill patients with COVID-19 [126].
Are AMDs always effective in viral infections?
Some studies suggest limited benefit from CQ/HCQ in the treatment of COVID-19 in general. There is evidence contradicting the use of these drugs in viral infections, underlying their adverse effect on viremia, which is explained by the drug-mediated delay of the adaptive immune response to infection. This suspect is evidenced by studies regarding the treatment of the chikungunya virus by AMDs in animals [127]. In the case of chikungunya infection, CQ administered as a preventive therapy increased the severity of symptoms and delayed the elimination of the virus in monkeys (probably by inhibiting the specific cellular response) [9]. Moreover, CQ increased the risk of late complications such as joint infection one year after the disease onset; however, when compared to placebo, there were no significant effects on the acute phase of fever [128]. Similarly, in humans, the adverse effects of CQ have been recorded during the treatment of acute-phase chikungunya fever [128].
CQ was ineffective in the prevention of influenza in humans [10] and the treatment of dengue [11]. It was also ineffective in eliminating SARS-CoV-1 infection in mice [14]. It may increase symptom severity and mortality in viral diseases, such as encephalomyocarditis, and it increases viral titers in various organs [129]. CQ might predispose patients with DM/PM to developing herpes zoster, particularly in women and patients with DM, independent of disease status, therapy, and demographic features [78].
A recent study analyzed the CQ regiment and showed that a high dose (600 mg CQ twice daily for 10 days or total dose of 12 g) is not sufficiently safe because it increased the QTc value to more than 500 ms (25%), and the trend toward higher mortality. Therefore, it should no longer be used in patients with a severe infection of SARS-CoV-2 [130].
Recent studies include larger groups of SARS-CoV-2 infected patients using AMDs, mainly HCQ. The study of Tang et al. analyzed 150 patients with mild COVID-19 who were treated with standard regimens with or without HCQ (ChiCTR2000029868). This study showed no significant differences between groups in conversion to negative SARS-CoV-2 RT-PCR and the degree of symptom resolution after 4 weeks. Moreover, the authors observed significantly more side effects in the HCQ arm (mainly no severe). Beneficial was the earlier normalization of CRP in the patients using HCQ with standard care, but the differences were not significant [101]. Another study analyzing adult outpatients (n = 423) investigated the efficacy of HCQ in the reduction of COVID-19 severity (March–May 2020, randomized, double-blind, placebo-controlled trial ClinicalTrials.gov: NCT04308668). The patients were treated with HCQ (first dose 800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg daily for 4 more days) or placebo. After 2 weeks of such treatment, the change in symptom severity did not differ between the HCQ and placebo groups. Adverse effects of HCQ were observed in 43% of patients (92 of 212) versus 22% receiving placebo (46 of 211, p < 0.001). The authors concluded that HCQ did not substantially reduce symptom severity in outpatients with early, mild COVID-19 [131].
Recently two studies analyzing the use of antimalarial drugs with azithromycin were published. The study of Cavalcanti et al. who analyzed the effect of therapy in three-group receiving standard care, standard care plus HCQ (2 × 400 mg/day), or standard care plus azithromycin (500 mg/day for 7 days) with HCQ (2 × 400 mg/day) in mild-to-moderate COVID-19. This study showed that HCQ ± azithromycin did not improve clinical status at 15 days compared with standard care (ClinicalTrials.gov number, NCT04322123). Moreover, prolonging the corrected QT interval and elevation of liver-enzyme levels were more frequent in patients receiving HCQ with or without azithromycin compared to standard care without these medications [132]. Conversely, the trial published by Arshad et al. showed that COVID-19 patients (n = 2541) treated with HCQ or azithromycin or a combination of two showed beneficial effects such as a reduction in COVID-19 associated mortality (when controlling for COVID-19 risk factors) [99]. Shamshirian et al., in meta-analysis describing HCQ use in COVID-19 treatment, revealed no clinical benefits to patients receiving HCQ with standard care [133].
On April 2020, the US CDC published information stating, “hydroxychloroquine and chloroquine are under investigation in clinical trials” [134]. However, in June 2020, FDA annulled the emergency use of CQ and HCQ to treat patients with COVID-19, considering the adverse effects of these medications [135]. Thus, even if AMDs might reduce the symptoms and improve the course of SARS-CoV-2 infection, some studies show that they have no benefits [124, 136] or are even hazardous to health [102].
Adverse effects of AMDs in rheumatic diseases
A high safety profile usually characterizes AMDs in rheumatic diseases; however, they are used in smaller doses (usually CQ 250–500 mg/day; HCQ 200–400 mg/day) than that of COVID-19 treatment [79]. In 250 mg/day, chloroquine provides a stable concentration in the range of 100–500 ng/ml. The onset of action of HCQ is observed after 4–6 weeks, but full efficacy can be assessed after 3 to 6 months [137, 138]. Nevertheless, of used dose, these medications can cause serious side effects, which should be monitored. The significant side effect of AMDs, mainly caused by CQ, is retinopathy. In prolonged and strict regimens, retinopathy depends on the cumulative dose [139]. Consequently, AMDs should be used in patients with no ocular problem, and the patients should be regularly examined by an ophthalmologist (a baseline eye exam and follow-up exam every 6–12 months are requisite) [140].
In addition to retinopathy, AMDs can cause other severe complications such as cardiac toxicity. Every patient should be strictly monitored and the therapy should be immediately ceased if there are changes in their electrocardiogram, such as prolonged QT interval, which is the risk of arrhythmia [123]. Other rare side effects of AMDs include nausea, agranulocytosis, and hemolysis in patients with the deficiency of glucose-6-phosphate [79]. Among the two available antimalarial drugs, HCQ is the one with the lowest retinal toxicity [4, 140], but CQ appears to be more effective [79].
The use of AMDs in smaller doses in rheumatic diseases is very safe, and severe complications such as antimalarial myopathy, cardiomyopathy, or maculopathy are rarely observed [141]. These medications are usually administered in smaller dosages for an extended period (not higher than 500 mg of HCQ per day and not more than 400 mg of CQ per day, usually 250 mg CQ or 200 mg HCQ is administered as a single evening dose). As a result, they show a high safety profile and are very well tolerated. Thus, in rheumatic diseases, toxicity related to AMDs is infrequent, mild, and usually reversible, with HCQ having a safer profile than that of CQ [4].
Adverse effects of AMDs in COVID-19 treatment
There are high differences between the dosage of AMDs in rheumatic and viral diseases. In SARS-CoV-2 infection, AMDs are used at a high dose to give the effect of drug saturation (600 mg CQ twice daily for 10 days (total dose 12 g)) [130]. This dose of CQ requires strict monitoring of the side effects, particularly in critically ill patients with renal and hepatic disorders (related to changed metabolic pathways and excretion of drug metabolites). Even AMDs are administered in adequate doses, and under close monitoring, their therapeutic window is narrow, which increases their risk of toxicity. Because AMDs have strong tissue tropism for the kidneys and liver, the risk of adverse effects will be higher in critically ill patients with renal or hepatic dysfunction than in patients with less severity [79].
AMDs can cause direct myocardial toxicity leading to cardiomyopathy and arrhythmias. They prolong the QT interval and increase the risk of arrhythmia [79]. Some studies did not confirm a significant effect of HCQ in patients with severe COVID-19 compared to patients without HCQ, but they show that 8 patients from 84 participants in the HCQ group (9.5%) discontinued HCQ after 4 days due to prolonged QT interval or first-degree atrioventricular block [124]. The same side effect was observed in a study of severe to critically ill patients with COVID-19 admitted in the ICU, who were administered with HCQ orally; however, it was withdrawn in 2 patients out of 13 participants [125]. The use of CQ with concurrent macrolides (azithromycin) and quinolone was not recommended as there is a risk of the prolonged QT interval [142]. Various studies have shown that HCQ does not reduce mortality or reduce the duration of the disease. This was particularly seen in a severely ill patient with advanced pneumonia [143]. In a previous study, the positive effect of AMDs has been described (mainly hypolipemic and hypoglycemic), but it also shows that they can cause hypoglycemia in severely ill patients [144].
Recent studies have shown that 30% of the patients with COVID-19 experience cardiac injury, which can further increase the risk of cardiomyopathy and arrhythmias. In such a situation, AMDs should be carefully used because the risk of cardiotoxic events is very high, particularly when they are used at a high dose [18, 19]. Because of the small difference in the therapeutic and toxic dose and life-threatening cardiovascular complications in the case of an overdose of CQ/HCQ, these drugs should only be used under strict medical supervision. In such cases, the choice of medication is crucial. HCQ has similar pharmacokinetics and mechanism of action as CQ but shows substantially fewer side effects [117, 145].
In June 2020, the US Food and Drug Administration (FDA) withdrew the conditional authorization for the use of AMDs in patients with emergency use in COVID-19 treated in hospitals unless they can participate in clinical trials. According to FDA, AMDs are unlikely to be effective in the treatment of patients with COVID-19 and that their benefits do not outweigh the risks identified in emergency use authorization (EUA), because of severe adverse events, mainly cardiological [146]. Moreover, the FDA issued a warning that AMDs reduce the antiviral activity of remdesivir. Because of the lack of clinical data, patients treated with the remdesivir for COVID-19 should not receive AMDs [147].
In conclusion, in the case of the prolonged QT interval, ocular problems, and neurological symptoms, AMDs should be withdrawn from the treatment of both rheumatic and viral diseases.
Prevention of toxicity of AMDs
Patients treated with AMDs at high doses and for a short time should be monitored for daily blood counts, electrolytes, and cardiac enzymes, as well as electrocardiogram monitoring [142] (Fig. 3). Before the start of the treatment, patients should be interviewed about visual changes during treatment; however, the American Academy of Ophthalmology does not recommend retinal screening before the short-term use of CQ [148]. In every case, the contraindications to HCQ therapy should be considered before ordering this medication (deficiency of glucose-6-phosphatase, QTc prolongation in the electrocardiogram, history of allergy to hydroxychloroquine, and the risk of retinopathy and cardiomyopathy) [17].
Conclusion
AMDs show therapeutic effects in various diseases, and recently, the evidence for their potential benefit continue to grow in autoimmune pathologies. The use of AMDs in connective tissue disorders is well established, and they reveal many beneficial effects (improve cutaneous and musculoskeletal symptoms, decrease the activity of the disease and organ damage, and reduce cardiovascular risk). In recent decades, many studies have reported the wide-ranging benefits of CQ and HCQ. However, the dose of AMDs in rheumatic diseases is much lower than that in SARS-CoV-2, and the time to achieve drug saturation is longer. The full therapeutic effect of these medications can be seen later, but the risk of severe complications is low. In addition, antimalarial myopathy, cardiomyopathy, or maculopathy are rarely observed.
AMDs change the pH of the endosomes during viral replication and they inhibit “cytokine storm” (mainly TNF-α and IL-6). Given that, both CQ and HCQ have been considered attractive candidates for the potential treatment of COVID-19. Recent studies have revealed both the beneficial and harmful effects of AMDs. The discrepancies in the use of CQ and HCQ might be due to various patient populations, which were characterized by a different course of the disease (mild vs. severe), the various dosage of the drug (400–1200 mg), COVID-19 association with other chronic diseases (mainly CVDs and respiratory disorders), lack of control group, and the use of CQ/HCQ with other drugs. Thus, the recommendations for the use of AMDs should be confirmed by randomized controlled trials. In a viral infection, the effect of AMDs is quickly required, and the drug saturation can be achieved by high doses. In such circumstances, the possibility of side effects is higher than that when a slow saturation is achieved in rheumatic diseases. The safety profile and dosage regiment of CQ/HCQ should be mainly assessed in critically ill patients with detailed analysis of other risk factors which may influence the effectiveness of AMDs (e.g., old age, multiple comorbidities, the synergistic effect of drugs, or delay therapy of COVID-19).
Therefore, we conclude that AMDs are safe and should be considered in the treatment of both rheumatic diseases and mild viral diseases. Furthermore, results from the ongoing trials are required to determine the effectiveness and safety of AMDs in treating COVID-19. Considering the fact that CQ and HCQ have excellent efficiency and low toxicity, they should be considered candidate drugs for post-exposure prophylaxis of SARS-CoV-2. However, controlled clinical trials are warranted to assess the extent of benefit and adverse effects of these medications in both the treatment and prevention of COVID-19.
References
Winzeler EA (2008) Malaria research in the post-genomic era. Nature 455:751–756
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388:2023–2038
Haładyj E, Sikora M, Felis-Giemza A, Olesińska M (2018) Antimalarials–are they effective and safe in rheumatic diseases? Reumatologia 56:164–173
Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA (2010) Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 69:20–28
Cairoli E, Rebella M, Danese N, Garra V, Borba EF (2012) Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus 21:1178–1182
Kyburz D, Brentano F, Gay S (2006) Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol 2:458–459
Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG (2012) Topical and systemic medications for the treatment of primary Sjögren’s syndrome. Nat Rev Rheumatol 8:399–411
Judson M, Barba B, Gobunsuy, (2013) Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Dev Ther 2013:325
Roques P, Thiberville SD, Dupuis-Maguiraga L, Lum FM, Labadie K, Martinon F et al (2018) Paradoxical effect of chloroquine treatment in enhancing Chikungunya virus infection. Viruses 268:1–18
Paton NI, Lee L, Xu Y, Ooi EE, Cheung YB, Archuleta S et al (2011) Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis 11:677–683
Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B et al (2010) A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Neglected Trop Dis 4:e785
Mahase E (2020) Covid-19: what treatments are being investigated? BMJ 368:m1252
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71:732–739
Barnard DL, Day CW, Bailey K (2006) Evaluation of immunomodulators, interferons and known in vitro SARS-CoV inhibitors for inhibition of SARS-CoV replication in BALB/c mice. Antivir Chem Chemother 17:275–284
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (2003) Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis 3:722–727
Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72–73
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56:105949
Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M et al (2020) Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3(4):e208857
Fihn SD, Perencevich E, Bradley SM (2020) Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Netw Open 3(4):e209035
He Y, Xu Y, Zhang C, Gao X, Dykema KJ, Martin KR et al (2011) Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Sci Signal 4(180):ra44
Valim V, Trevisani VF, Pasoto SG, Serrano EV, Ribeiro SL, Fidelix TS et al (2015) Recommendations for the treatment of Sjogren’s syndrome. Rev Bras Reumatol 55:446–457
Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A, Egurbide MV, Aguirre C (2009) Predictors of major infections in systemic lupus erythematosus. Arthritis Res Ther 11:R109
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16(3):155–166
Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW et al (2004) Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA 101:5598–5603
Willis R, Seif AM, McGwin G Jr, Martinez-Martinez LA, González EB, Dang N et al (2012) Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 21:830–835
Petri M (2011) Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep 13:77–80
James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR et al (2007) Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 16:401–409
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD et al (2012) American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64:797–808
Clowse ME, Magder L, Witter F, Petri M (2006) Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 54:3640–3647
Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A 75(7):3327–3331
Ziegler HK, Unanue ER (1982) Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A 79(1):175–8
Wozniacka A, Lesiak A, Boncela J, Smolarczyk K, McCauliffe DP, Sysa-Jedrzejowska A (2008) The influence of antimalarial treatment on IL-1beta, IL-6 and TNF-alpha mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br J Dermatol 159:1124–1130
Suarez-Almazor ME, Belseck E, Shea B, Homik J, Wells G, Tugwell P (2000) Antimalarials for treating rheumatoid arthritis. Cochrane Database Syst Rev 4:CD000959
Vivino FB, Carsons SE, Foulks G, Daniels TE, Parke A, Brennan MT, Forstot SL, Scofield RH, Hammitt KM (2016) New treatment guidelines for sjogren’s disease. Rheum Dis Clin N Am 42(3):531–551
Mumcu G, Bicakcigil M, Yilmaz N, Ozay H, Karacayli U, Cimilli H, Yavuz S (2013) Salivary and serum B-cell activating factor (BAFF) levels after hydroxychloroquine treatment in primary Sjogren’s syndrome. Oral Health Prev Dent 11(3):229–234
Yavuz S, Asfuroglu E, Bicakcigil M, Toker E (2011) Hydroxychloroquine improves dry eye symptoms of patients with primary Sjogren’s syndrome. Rheumatol Int 31(8):1045–1049
Carsons SE, Vivino FB, Parke A, Carteron N, Sankar V, Brasington R et al (2017) Treatment guidelines for rheumatologic manifestations of Sjögren’s syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res 69:517–527
Gottenberg JE, Ravaud P, Puechal X, Le Guern V, Sibilia J, Goeb V et al (2014) Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: the JOQUER randomized clinical trial. JAMA 312:249–258
Yoon CH, Lee HJ (2016) Effect of hydroxychloroquine treatment on dry eyes in subjects with primary Sjogren’s syndrome: a double-blind randomized control study. J Korean Med Sci 31(7):1127–1135
Kalia S, Dutz JP (2007) New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 20(4):160–174
Doherty CB, Rosen T (2008) Evidence-based therapy for cutaneous sarcoidosis. Drugs 68:1361–1383
Jones E, Callen JP (1990) Hydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomas. J Am Acad Dermatol 23(3 Pt 1):487–489
Baltzan M, Mehta S, Kirkham TH, Cosio MG (1999) Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med 160(1):192–197
Hilderson I, Van Laecke S, Wauters A, Donck J (2014) Treatment of renal sarcoidosis: is there a guideline? Overview of the different treatment options. Nephrol Dialysis Transplant 29:1841–1847
Adams JS, Diz MM, Sharma OP (1989) Effective reduction in the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Ann Internal Med 111:437–438
Sweiss NJ, Patterson K, Sawaqed R et al (2010) Rheumatologic manifestations of sarcoidosis. Semin Respir Crit Care Med 31:463–473
Arthritis in Sarcoidosis Group (ASG), Agarwal V, Agrawal V, Aggarwal A, Aggarwal P, Chowdhury AC et al (2018) Arthritis in sarcoidosis: a multicentric study from India. Int J Rheum Dis 21:1728–1733. https://doi.org/10.1111/1756-185X.13349
Fritz D, van de Beek D, Brouwer MC (2016) Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol 16:220
Ang GC, Werth VP (2005) Combination antimalarials in the treatment of cutaneous dermatomyositis. A retrospective study. Arch Dermatol 141(7):855–859
Woo TY, Callen JP, Voorhees JJ, Bickers DR, Hanno R, Hawkins C (1984) Cutaneous lesions of dermatomyositis are improved by hydroxychloroquine. J Am Acad Dermatol 10(4):592–600
Cavagna L, Monti S, Caporali R, Gatto M, Iaccarino L, Doria A (2017) How I treat idiopathic patients with inflammatory myopathies in the clinical practice. Autoimmun Rev 16(10):999–1007
Yu C, Gershwin ME, Chang C (2014) Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 48–49:10–13
Lam NC, Ghetu MV, Bieniek ML (2016) Systemic lupus erythematosus: primary care approach to diagnosis and management. Am Fam Physician 94:284–294
Davis LS, Reimold AM (2017) Research and therapeutics—traditional and emerging therapies in systemic lupus erythematosus. Rheumatology (Oxford) 56(Suppl 1):i100–i113
Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B et al (2017) European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 76:1788–1796
Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM et al (2007) Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 66:1168–1172
Canadian hydroxychloroquine study group (1991) A Randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus . N Engl J Med 324:150–154. https://doi.org/10.1056/NEJM199101173240303
Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers Ch et al (2016) The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 75:795–810
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81:646–656
Lillegraven S, Kvien TK (2007) Measuring disability and quality of life in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 21:827–840
Kim D, Choi CB, Lee J, Cho SK, Won S, Bang SY et al (2017) Impact of early diagnosis on functional disability in rheumatoid arthritis. Korean J Intern Med 32:738–746
Gonzalez-Lopez L, Gamez-Nava JI, Jhangri G, Russell AS, Suarez-Almazor ME (2000) Decreased progression to rheumatoid arthritis or other connective tissue diseases in patients with palindromic rheumatism treated with antimalarials. J Rheumatol 27:41–46
Hanonen P, Mottonen T, Oka M (1987) Treatment of palindromic rheumatism with chloroquine. BMJ 294:1289
Iredale J, Fieger H, Wainer IW (1993) Determination of the stereoisomers of hydroxychloroquine and its major metabolites in plasma and urine following a single oral administration of racemic hydroxychloroquine. Semin Arthritis Rheum 23:74–81
O’Dell JR, Haire CE, Erikson N, Drymalski W, Palmer W, Eckhoff PJ et al (1996) Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 334:1287–1291
Calgüneri M, Pay S, Caliskaner Z, Apraş S, Kiraz S, Ertenli I et al (1999) Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clin Exp Rheumatol 17:699–704
Scofield RH (2011) Vasculitis in Sjögren’s Syndrome. Curr Rheumatol Rep 13(6):482–488
Seror R, Bootsma H, Saraux A, Bowman SJ, Theander E, Brun JG et al (2016) Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis 75:382–389
Liang Y, Yang Z, Qin B, Zhong R (2014) Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis 73(6):1151–1156
Brito-Zerón P, Retamozo S, Gheitasi H, Ramos-Casals M (2016) Treating the underlying pathophysiology of primary Sjögren syndrome: recent advances and future prospects. Drugs 76:1601–1623
Lundberg I, Chung Y (2000) Treatment and investigation of idiopathic inflammatory myopathies. Rheumatology (Oxford) 39(1):7–17
Schmidt J (2018) Current classification and management of inflammatory myopathies. J Neuromuscul Dis 5(2):109–129
Dalakas MC (2015) Inflammatory muscle diseases. N Engl J Med 372(18):1734–1747
Bendewald MJ, Wetter DA, Li X, Davis MDP (2010) Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County Minnesota. Arch Dermatol 146(1):26–30
Carstens PO, Schmidt J (2014) Diagnosis, pathogenesis and treatment of myositis: recent advances. Clin Exp Immunol 175(3):349–358
Sato JO, Sallum AM, Ferriani VP, Marini R, Sacchetti SB, Okuda EM et al (2009) Rheumatology Committee of the São Paulo Paediatrics Society. A Brazilian registry of juvenile dermatomyositis: onset features and classification of 189 cases. Clin Exp Rheumatol 27:1031–1038
Abdel-Hamid H, Oddis CV, Lacomis D (2008) Severe hydroxychloroquine myopathy. Muscle Nerve 38(3):1206–1210
Cunha GF, Souza FH, Levy-Neto M, Shinjo SK (2013) Chloroquine diphosphate: a risk factor for herpes zoster in patients with dermatomyositis/polymyositis. Clinics (Sao Paulo) 68:621–627
Schrezenmeier E, Dorner T (2020) Mechanisms of action of hydroxychloroquine andchloroquine: implications for rheumatology. Nat Rev Rheumatol 16(3):155–166
Sakthivel P, Bruder D (2017) Mechanism of granuloma formation in sarcoidosis. Curr Opin Hematol 24(1):59–65
Arkema EV, Cozier YC (2018) Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis 9(11):227–240
Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y, Müller-Quernheim J (2014) Sarcoidosis. Lancet 383(9923):1155–1167
Boelaert JR, Piette J, Sperber K (2001) The potential place of chloroquine in the treatment of HIV-1-infected patients. J Clin Virol 20:137–140
Devaux ChA, Rolain JM, Colson Ph, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 55:105938
Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H (2008) Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res 77:150–2
Olofsson S, Kumlin U, Dimock K, Arnberg N (2005) Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis 5:184–188
Shen L, Yang Y, Ye F, Liu G, Desforges M, Talbot PJ et al (2016) Safe and sensitive antiviral screening platform based on recombinant human coronavirus OC43 expressing the luciferase reporter gene. Antimicrob Agents Chemothe 60:5492–5503
Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G et al (2008) SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 18:290–301
Savarino A, Gennero L, Sperber K, Boelaert JR (2001) The anti-HIV-1 activity of chloroquine. J Clin Virol 20:131–135
Sperber K, Louie M, Kraus T, Proner J, Sapira E, Lin S et al (1995) Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther 17:622–636
Harley CA, Dasgupta A, Wilson DW (2001) Characterization of herpes simplex virus–containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J Virol 75:1236–1251
Randolph VB, Winkler G, Stollar V (1990) Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174:450–458
Farias KJ, Machado PR, de Almeida Junior RF, de Aquino AA, da Fonseca BA (2014) Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol Immunol 58:318–326
Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C (2013) Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 23:300–302
Perrier A, Bonnin A, Desmarets L, Danneels A, Goffard A, Rouillé Y et al (2019) The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J Biol Chem 294:14406–14421
Li R, Qiao S, Zhang G (2020) Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect 80:469–496
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG et al (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69
Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B, Zhang Z (2020) Efficacy of hydroxychloroquine in patients with COVID-19 results of a randomized clinical trial. medRxiv. https://doi.org/10.1101/2020.03.22.20040758
Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K et al (2020) Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 97:396–403
Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR et al (2020) Effect of Hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv 2020.07.15.20151852
Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W et al (2020) Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv 2020.04.10.20060558
Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, Ambati J (2020) Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Medicine 2020. https://doi.org/10.1016/j.medj.2020.06.001
Mitja O, Ubals M, Corbacho M, Alemany A, Suner C, Tebe C et al (2020) A cluster-randomized trial of hydroxychloroquine as prevention of Covid-19 transmission and disease. medRxiv 2020.07.20.20157651
Kwiek JJ, Haystead TA, Rudolph J (2004) Kinetic mechanism of quinone oxidore-ductase 2 and its inhibition by the antimalarial quinolines. Biochemistry 43:4538–4547
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al (2020) Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. https://doi.org/10.1101/2020.01.22.914952
Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A (2006) New insights into the antiviral effects of chloroquine. Lancet Infect Dis 6(2):67–69
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel Coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Bakkers MJG, Lang Y, Feistsma LJ, Hulswit RJG, de Poot SAH, van Vliet ALW et al (2017) Betacoronavirus adaptation to humans involved progressive loss of hemagglutinin-esterase lectin activity. Cell Host Microbe 21:356–366
Wang PH, Cheng Y (2020) Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. https://doi.org/10.1101/2020.02.24.963348
Millet JK, Whittaker GR (2015) Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res 202:120–134
Burkard C, Verheije MH, Wicht O, van Kasteren SI, van Kuppeveld FJ, Haag-mans BL et al (2014) Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog 10:e1004502
Simmons G, Bertram S, Glowacka I, Steffen I, Chaipan C, Agudelo J et al (2011) Different host cell proteases activate the SARS-coronavirus spike-protein for cell–cell and virus–cell fusion. Virology 413:265–274
An J, Woodward JJ, Lai W, Minie M, Sun X, Tanaka L et al (2018) Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in Trex1-deficient mice. Arthritis Rheumatol 70(11):1807–1819
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY (2008) Priming for T helper type 2 differentiation by interleukin 2-mediated induction of IL-4 receptor ? Chain expression. Nat Immunol 9:1288–1296
Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M (2004) In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 323(1):264–268
Shippey EA, Wagler VD, Collamer AN (2018) Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med 85:459–467
Shanghai New Coronary Virus Disease Clinical Treatment Expert Group, Shanghai 2019 Coronary Virus Disease Comprehensive Treatment Expert Consensus, Chinese Journal of Infectious Diseases (2020) Expert group on clinical treatment of new coronavirus disease in Shanghai China. J Infect Dis 38. https://doi.org/10.3760/cma.j.issn.1000-6680.2020.0016
Zhou D, Dai SM, Tong Q (2020) COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 75:1667–1670
Ben-Zvi I, Kivity S, Shoenfeld LP, Y, (2012) Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol 42:145–153
Nosal R, Jancinova V, Petrikova M (1995) Chloroquine inhibits stimulated platelets at the arachidonic acid pathway. Thromb Res 77:531–542
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18:1094–1099
Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L et al (2020) No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients withsevere COVID-19 infection. Med Mal Infect 50:384
Mahevas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C et al (2020) Noevidence of clinical efficacy of hydroxychloroquine in patients hospitalizedfor COVID-19 infection with oxygen requirement: results of a study usingroutinely collected data to emulate a target trial. MedRxiv 2020. https://doi.org/10.1101/2020.04.10.20060699
Perinel S, Launay M, Botelho-Nevers É, Diconne É, Louf-Durier A, Lachand R et al (2020) Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis 71(16):2227–2229. https://doi.org/10.1093/cid/ciaa394
Lombardy Section Italian Society Infectious and Tropical Diseases (2020) Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med 28(2):143–152
Touret F, de Lamballerie X (2020) Of chloroquine and COVID-19. Antiviral Res 177:104762. https://doi.org/10.1016/j.antiviral.2020.104762
de Lamballerie X, Boisson V, Reynier JC, Enault S, Charrel RN, Flahault A, Roques P, Le Grand R (2008) On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis 8(6):837–839. https://doi.org/10.1089/vbz.2008.0049
Sidhu GS, Gaddipati JP, Vogel SN, Maheshwari RK (1999) Acceleration of viral replication and up-regulation of cytokine levels by antimalarials: implications in malaria-endemic areas. Am J Trop Med Hyg 61(2):180–186
Borba MGS, Val FdA, Sampaio VS, Alexandre MAA, Melo GC, Brito M et al (2020) Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb cl. MedRxiv 2020. https://doi.org/10.1101/2020.04.07.20056424.04.07.20056424
Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM et al (2020) Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med M20–M4207. https://doi.org/10.7326/M20-4207
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A et al (2020) Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2019014
Shamshirian A, Hessami A, Heydari K, Alizadeh-Navaei R, Ebrahimzadeh MA, Ghasemian R et al (2020) Hydroxychloroquine versus COVID-19: a rapid systematic review and meta-analysis. medRxiv 20065276. https://doi.org/10.1101/2020.04.14
Stanglin D (2020) CDC Website Drops Guidance, Anecdotal Data on Trump-Backed Hydroxychloroquine as COVID-19 Treatment. Available from: https://us.yahoo.com/news/cdc-website-drops-guidance-anecdotal-165821932.html . Last accessed on 2020 Apr 08
fda. gov. (2020) Letter revoking EUA for chloroquine phosphate and hydroxychloroquine sulfate, 6/15/2020. June 2020. Available at: https://www.fda.gov/media/138945/download. Accessed on 15th June 2020
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G et al (2020) Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 382:2411–2418
Furst DE, Lindsley H, Baethge B et al (1999) Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension. Arthritis Rheum 42:357–365
Götestam Skorpen C, Hoeltzenbein M, Tincani A et al (2016) The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 75:795–810
Bernstein HN (1991) Ocular safety of hydroxychloroquine. Ann Ophthalmol 23:292–296
Bernstein HN (1983) Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med 75(1):25–34
Tselios K, Deeb M, Gladman DD, Harvey P, Urowitz MB (2018) Antimalarial-induced cardiomyopathy: a systematic review of the literature. Lupus 27(4):591–599
Multicenter Collaboration Group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia (2020) Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43(3):185–188. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.03.009. Chinese
Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C et al (2020) Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 14(369):m1844. https://doi.org/10.1136/bmj.m1844
Costedoat-Chalumeau N, Dunogue B, Leroux G, Morel N, Jallouli M, Le Guern V et al (2015) A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol 49(3):317–326
Biot C, Daher W, Chavain N, FandeurT KJ, Dive D, De Clercq E (2006) Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem 49(9):2845–2849
FDA News Release: Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and?utm_campaign=FDA%20Revokes%20Emergency%20Use%20Authorization%20for%20Chloroquine%20and%20Hydroxychloroquine&utm_medium=email&utm_source=Eloqua
FDA News Release: Coronavirus (COVID-19) Update: FDA warns of newly discovered potential drug interaction that may reduce effectiveness of a COVID-19 Treatment Authorized for Emergency Use. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-newly-discovered-potential-drug-interaction-may-reduce?utm_campaign=FDA%20Warns%20of%20Newly%20Discovered%20Potential%20Drug%20Interaction%20That%20May%20Reduce%20Effectiveness&utm_medium=email&utm_source=Eloqua
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF (2016) American Academy of Ophthalmology Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 123(6):1386–94
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grygiel-Górniak, B. Antimalarial drugs—are they beneficial in rheumatic and viral diseases?—considerations in COVID-19 pandemic. Clin Rheumatol 41, 1–18 (2022). https://doi.org/10.1007/s10067-021-05805-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05805-5