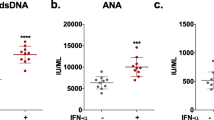
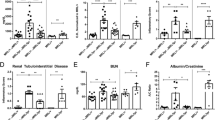
Abstract
Systemic lupus erythematosus (SLE) that involves neurological complications is known as neuropsychiatric systemic lupus erythematosus (NPSLE). Research in humans is difficult due to the disease’s great heterogeneity. Animal models are a resource for new discoveries. In this review, we examine experimental models of lupus that present neuropsychiatric manifestations. Spontaneous animal models such as NZB/W F1 and MRL/lpr are commonly used in NPSLE research; these models present few SLE symptoms compared to induced animal models, such as pristane-induced lupus (PIL). The PIL model is known to present eight of the main clinical and laboratory manifestations of SLE described by the American College of Rheumatology. Many cytokines associated with NPSLE are expressed in the PIL model, such as IL-6, TNF-α, and IFN. However, to date, NPSLE manifestations have been poorly studied in the PIL model. In this review article, we discuss whether the PIL model can mimic neuropsychiatric manifestations of SLE.
Key Points • PIL model have a strong interferon signature. • Animals with PIL express learning and memory deficit. |



Similar content being viewed by others
References
Shaheen VM, Satoh M, Richards HB et al (1999) Immunopathogenesis of environmentally induced lupus in mice. Environ Health Perspect 107(Suppl):723–727. https://doi.org/10.1289/ehp.99107s5723
Kivity S, Agmon-Levin N, Zandman-Goddard G et al (2015) Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med 13:43. https://doi.org/10.1186/s12916-015-0269-8
Wen J, Stock AD, Chalmers SA, Putterman C (2016) The role of B cells and autoantibodies in neuropsychiatric lupus. Autoimmun Rev 15:890–895
Aranow C, Diamond B, Mackay M (2012) Pathogenesis of the nervous system. In: Dubois’ lupus erythematosus and related syndromes: eighth edition. Pp 363–367
Rizos T, Siegelin M, Hähnel S et al (2009) Fulminant onset of cerebral immunocomplex vasculitis as first manifestation of neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus 18:361–363. https://doi.org/10.1177/0961203308097448
Vo A, Volpe BT, Tang CC et al (2014) Regional brain metabolism in a murine systemic lupus erythematosus model. J Cereb Blood Flow Metab 34:1315–1320. https://doi.org/10.1038/jcbfm.2014.85
Kaul A, Gordon C, Crow MK et al (2016) Systemic lupus erythematosus. Nat Rev Dis Prim 2:16039. https://doi.org/10.1038/nrdp.2016.39
Shao W-H, Cohen PL (2011) Disturbances of apoptotic cell clearance in SLE. Arthritis Res Ther 13:202. https://doi.org/10.1186/ar3206
Theofilopoulos AN, Kono DH, Beutler B, Baccala R (2011) Intracellular nucleic acid sensors and autoimmunity. J Interf Cytokine Res 31:867–886. https://doi.org/10.1089/jir.2011.0092
Nelson P, Rylance P, Roden D et al (2014) Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus 23:596–605. https://doi.org/10.1177/0961203314531637
Wolf SJ, Estadt SN, Theros J et al (2019) Ultraviolet light induces increased T cell activation in lupus-prone mice via type I IFN-dependent inhibition of T regulatory cells. J Autoimmun 103:102291. https://doi.org/10.1016/j.jaut.2019.06.002
Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH (2017) Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31:306–320. https://doi.org/10.1016/j.berh.2017.09.005
Yap DYH, Lai KN (2010) Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol 2010:365083. https://doi.org/10.1155/2010/365083
Sule S, Rosen A, Petri M et al (2011) Abnormal production of pro- and anti-inflammatory cytokines by lupus monocytes in response to apoptotic cells. PLoS One 6:e17495. https://doi.org/10.1371/journal.pone.0017495
Baechler EC, Batliwalla FM, Karypis G et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100:2610–2615. https://doi.org/10.1073/pnas.0337679100
Dall’Era MC, Cardarelli PM, Preston BT et al (2005) Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis 64:1692–1697. https://doi.org/10.1136/ard.2004.033753
Pieterse E, van der Vlag J (2014) Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol 5. https://doi.org/10.3389/fimmu.2014.00164
Ginzler E, Tayar J (2013) Systemic lupus erythematosus. Am Coll Rheumatol:1–6. https://doi.org/10.1007/SpringerReference_61618
Bertsias GK, Ioannidis JPA, Aringer M et al (2010) EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 69:2074–2082. https://doi.org/10.1136/ard.2010.130476
Liang MH, Corzillius M, Bae SC et al (1999) The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 42:599–608. https://doi.org/10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F
Lacomis D, Živković SA (2007) Approach to vasculitic neuropathies. J Clin Neuromuscul Dis 9:265–276
Sciascia S, Bertolaccini ML, Roccatello D et al (2014) Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol 261:1706–1714. https://doi.org/10.1007/s00415-014-7406-8
Wallace DJ, Hahn BH (2018) Dubois’ lupus erythematosus and related syndromes
Hanly JG (2014) Diagnosis and management of neuropsychiatric SLE. Nat. Rev. Rheumatol
Stock AD, Wen J, Putterman C (2013) Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front Immunol 4. https://doi.org/10.3389/fimmu.2013.00484
Ballok DA, Millward JM, Sakic B (2003) Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining. Brain Res. https://doi.org/10.1016/S0006-8993(02)03980-X
De Boer AG, Gaillard PJ (2006) Blood-brain barrier dysfunction and recovery. J Neural Transm 113:455–462. https://doi.org/10.1007/s00702-005-0375-4
Sharif Y, Jumah F, Coplan L et al (2018) Blood brain barrier: a review of its anatomy and physiology in health and disease. Clin Anat 31:812–823. https://doi.org/10.1002/ca.23083
Bonkowski D, Katyshev V, Balabanov RD, et al (2011) The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8
Patel JP, Frey BN (2015) Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast 2015
Jacob A, Hack B, Chen P et al (2011) C5a/CD88 signaling alters blood-brain barrier integrity in lupus through nuclear factor-κB. J Neurochem 119:1041–1051. https://doi.org/10.1111/j.1471-4159.2011.07490.x
Jafri K, Patterson SL, Lanata C (2017) Central nervous system manifestations of systemic lupus erythematosus. Rheum Dis Clin N Am 43:531–545. https://doi.org/10.1016/j.rdc.2017.06.003
Kowal C, DeGiorgio LA, Lee JY et al (2006) Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci 103:19854–19859. https://doi.org/10.1073/pnas.0608397104
Gelb S, Stock AD, Anzi S et al (2018) Mechanisms of neuropsychiatric lupus: the relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J Autoimmun 91:34–44. https://doi.org/10.1016/j.jaut.2018.03.001
Gelb S, Stock AD, Anzi S et al (2018) Mechanisms of neuropsychiatric lupus: the relative roles of the blood-CSF versus blood-brain barrier HHS public access. J Autoimmun 91:34–44. https://doi.org/10.1016/j.jaut.2018.03.001
Blank T, Prinz M (2017) Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 65:1397–1406. https://doi.org/10.1002/glia.23154
Rice GI, Rodero MP, Crow YJ (2015) Human disease phenotypes associated with mutations in TREX1. J Clin Immunol 35:235–243. https://doi.org/10.1007/s10875-015-0147-3
Kisla Ekinci RM, Balci S, Bisgin A et al (2017) A homozygote TREX1 mutation in two siblings with different phenotypes: chilblains and cerebral vasculitis. Eur J Med Genet 60:690–694. https://doi.org/10.1016/j.ejmg.2017.09.004
Appenzeller S, Costallat LTL (2007) Central nervous system manifestations in systemic lupus erythematosus. Curr Rheumatol Rev 3:205–214. https://doi.org/10.2174/157339707781387572
Lipsky PE (2001) Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 2:764–766. https://doi.org/10.1038/ni0901-764
Perry D, Sang A, Yin Y et al (2011) Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011:1–19. https://doi.org/10.1155/2011/271694
Richard ML, Gilkeson G (2018) Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med 5:e000199. https://doi.org/10.1136/lupus-2016-000199
Theofilopoulos AN, Dixon FJ (1985) Murine models of systemic lupus erythematosus. Adv Immunol 269–390:v9
Jeltsch-David H, Muller S (2014) Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol 10:579–596. https://doi.org/10.1038/nrneurol.2014.148
Andrews BS, Eisenberg RA, Theofilopoulos AN et al (1978) Spontaneous murine lupus-like syndromes: clinical and immunopathological manifestations in several strains*. J Exp Med 148:1198–1215. https://doi.org/10.1084/jem.148.5.1198
Freitas EC, de Oliveira MS, Monticielo OA (2017) Pristane-induced lupus: considerations on this experimental model. Clin Rheumatol 36:2403–2414. https://doi.org/10.1007/s10067-017-3811-6
Luciano-Jaramillo J, Sandoval-García F, Vázquez-Del Mercado M et al (2019) Downregulation of hippocampal NR2A/2B subunits related to cognitive impairment in a pristane-induced lupus BALB/c mice. PLoS One 14:e0217190. https://doi.org/10.1371/journal.pone.0217190
Nacionales DC, Kelly-Scumpia KM, Lee PY et al (2007) Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum 56:3770–3783. https://doi.org/10.1002/art.23023
Correa Freitas E, Evelyn Karnopp T, de Souza Silva JM et al (2019) Vitamin D supplementation ameliorates arthritis but does not alleviates renal injury in pristane-induced lupus model. Autoimmunity 52:69–77. https://doi.org/10.1080/08916934.2019.1613383
Kier AB (1990) Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J Comp Pathol 102:165–177. https://doi.org/10.1016/S0021-9975(08)80122-3
Gao HX, Campbell SR, Cui MH et al (2009) Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. https://doi.org/10.1016/j.jneuroim.2008.11.009
Gulinello M, Putterman C (2011) The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus. J Biomed Biotechnol 2011:1–15. https://doi.org/10.1155/2011/207504
Šakić B, Szechtman H, Talangbayan H et al (1994) Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav 56:609–617. https://doi.org/10.1016/0031-9384(94)90309-3
He Y-YY, Yan YY, Zhang H-FF et al (2016) Methyl salicylate 2-O-β-D-lactoside alleviates the pathological progression of pristane-induced systemic lupus erythematosus-like disease in mice via suppression of inflammatory response and signal transduction. Drug Des Devel Ther Volume 10:3183–3196. https://doi.org/10.2147/DDDT.S114501
Wu WM, Lin BF, Su YC et al (2000) Tamoxifen decreases renal inflammation and alleviates disease severity autoimmune NZB/W F1 mice. Scand J Immunol 52:393–400. https://doi.org/10.1046/j.1365-3083.2000.00789.x
Zhuang H, Szeto C, Han S et al (2015) Animal models of interferon signature positive lupus. Front Immunol 6. https://doi.org/10.3389/fimmu.2015.00291
Tang B, Matsuda T, Akira S et al (1991) Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol 3:273–278
Tsai C-Y, Wu T-H, Huang S-F et al (1995) Abnormal splenic and thymic IL-4 and TNF-α expression in MRL-lpr/lpr mice. Scand J Immunol 41:157–163. https://doi.org/10.1111/j.1365-3083.1995.tb03548.x
Li P, Lin W, Zheng X (2014) IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation 37:824–832. https://doi.org/10.1007/s10753-013-9802-0
Murphy ED, Roths JB (1979) A y chromosome associated factor in strain bxsb producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum 22:1188–1194. https://doi.org/10.1002/art.1780221105
Satoh M (1994) Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med 180:2341–2346. https://doi.org/10.1084/jem.180.6.2341
Satoh M, Kumar A, Kanwar YS, Reeves WH (1995) Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci U S A 92:10934–10938. https://doi.org/10.1073/pnas.92.24.10934
Satoh M, Hamilton KJ, Ajmani AK et al (1996) Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J Immunol 157:3200–3206
Satoh M, Richards HB, Shaheen VM et al (2000) Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol 121:399–405. https://doi.org/10.1046/j.1365-2249.2000.01276.x
Richards HB (2002) Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med 188:985–990. https://doi.org/10.1084/jem.188.5.985
Reeves WH, Lee PY, Weinstein JS et al (2009) Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol 30:455–464. https://doi.org/10.1016/j.it.2009.06.003
Kalim H, Pratama MZ, Nugraha AS et al (2018) Regulatory T cells compensation failure cause the dysregulation of immune response in pristane induced lupus mice model. Malays J Med Sci 25:17–26. https://doi.org/10.21315/mjms2018.25.3.3
Schwartz N, Stock AD, Putterman C (2019) Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 15:137–152
Bortoluzzi A, Piga M, Silvagni E et al (2019) Peripheral nervous system involvement in systemic lupus erythematosus: a retrospective study on prevalence, associated factors and outcome. Lupus 28:465–474. https://doi.org/10.1177/0961203319828499
Bendorius M, Po C, Muller S, Jeltsch-David H (2018) From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci 19:3588. https://doi.org/10.3390/ijms19113588
Helyer BJ, Howie JB (1963) Renal disease associated with positive lupus erythematosus tests in a crossbred strain of mice. Nature. https://doi.org/10.1038/197197a0
Drake CG, Rozzo SJ, Hirschfeld HF et al (1995) Analysis of the New Zealand Black contribution to lupus-like renal disease. Multiple genes that operate in a threshold manner. J Immunol 54:2441–2447
Bracci-Laudiero L, Aloe L, Lundeberg T, et al (1999) Altered levels of neuropeptides characterize the brain of lupus prone mice. Neurosci Lett
Ballok DA (2007) Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res Rev 54:67–79. https://doi.org/10.1016/j.brainresrev.2006.12.003
Murphy ED, Roths JB (1979) A y chromosome associated factor in strain bxsb producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum https://doi.org/10.1002/art.1780221105
Li Y, Eskelund AR, Zhou H et al (2015) Behavioral deficits are accompanied by immunological and neurochemical changes in a mouse model for neuropsychiatric lupus (NP-SLE). Int J Mol Sci 16:15150–15171. https://doi.org/10.3390/ijms160715150
Ballok DA, Woulfe J, Sur M et al (2004) Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus 14:649–661
Gilkeson GS (2015) Complement-targeted therapies in lupus. Curr Treat Options Rheumatol 1:10–18. https://doi.org/10.1007/s40674-014-0009-9
Alexander JJ, Bao L, Jacob A et al (2003) Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim Biophys Acta Mol basis Dis 1639:169–176. https://doi.org/10.1016/j.bbadis.2003.09.005
Ma X, Foster J, Sakic B (2006) Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J Neuroimmunol 179:26–36. https://doi.org/10.1016/j.jneuroim.2006.06.023
Mike EV, Makinde HM, Der E et al (2018) Neuropsychiatric systemic lupus erythematosus is dependent on sphingosine-1-phosphate signaling. Front Immunol 9:2189. https://doi.org/10.3389/fimmu.2018.02189
Dean GS (2000) Cytokines and systemic lupus erythematosus. Ann Rheum Dis 59:243–251. https://doi.org/10.1136/ard.59.4.243
Arisi GM (2014) Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav 38:43–47
Dinan TG, Dinan T (2009) Inflammatory markers in depression. Curr Opin Psychiatry 22:32–36
Tomita M, Khan RL, Blehm BH, Santoro TJ (2004) The potential pathogenetic link between peripheral immune activation and the central innate immune response in neuropsychiatric systemic lupus erythematosus. Med Hypotheses 62:325–335. https://doi.org/10.1016/j.mehy.2003.10.009
Han J-HH, Umiker BR, Kazimirova AA et al (2014) Expression of an anti-RNA autoantibody in a mouse model of SLE increases neutrophil and monocyte numbers as well as IFN-I expression. Eur J Immunol 44:215–226. https://doi.org/10.1002/eji.201343714
Shi D, Tian T, Yao S et al (2018) FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav Immun 70:293–304. https://doi.org/10.1016/j.bbi.2018.03.009
Mike EV, Makinde HM, Gulinello M et al (2019) Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. J Autoimmun 96:59–73. https://doi.org/10.1016/j.jaut.2018.08.005
Cuda CM, Misharin AV, Gierut AK et al (2014) Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol 192:5548–5560. https://doi.org/10.4049/jimmunol.1400122
Makinde HM, Winter DR, Procissi D et al (2020) A novel microglia-specific transcriptional signature correlates with behavioral deficits in neuropsychiatric lupus. Front Immunol 11. https://doi.org/10.3389/fimmu.2020.00230
Nacionales DC, Kelly KM, Lee PY et al (2006) Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane). Am J Pathol 168:1227–1240. https://doi.org/10.2353/ajpath.2006.050125
Alexander JJ, Jacob A, Bao L et al (2005) Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J Immunol 175:8312–8319. https://doi.org/10.4049/jimmunol.175.12.8312
Lee PY, Weinstein JS, Nacionales DC et al (2014) A novel type I IFN-producing cell subset in murine lupus. J Immunol 180:5101–5108. https://doi.org/10.4049/jimmunol.180.7.5101
Tomita M, Holman BJ, Santoro TJ (2001) Aberrant cytokine gene expression in the hippocampus in murine systemic lupus erythematosus. Neurosci Lett 302:129–132. https://doi.org/10.1016/S0304-3940(01)01679-2
Tomita M, Holman BJ, Williams LS et al (2001) Cerebellar dysfunction is associated with overexpression of proinflammatory cytokine genes in lupus. J Neurosci Res 64:26–33. https://doi.org/10.1002/jnr.1050
Jeltsch-David H, Muller S (2014) Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmun Rev 13:963–973. https://doi.org/10.1016/j.autrev.2014.08.015
Murphy E, Roths J (1978) Autoimmunity and lymphoproliferation. Induction by mutant gene lpr, and acceleration by a male-associated factor in strain BXSB mice. In: Rose N, Bigazzi P, Warner N (eds) Genetic control of autoimmune disease. Elsevier, New York, pp 207–221
Furukawa F, Hamashima Y (1982) Lupus band test in New Zealand mice and MRL mice. J Dermatol 9:467–471. https://doi.org/10.1111/j.1346-8138.1982.tb01091.x
Acknowledgments
We acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, for a scholarship offered to Thaís Evelyn Karnopp and the Pró-Reitoria de Pesquisa (PROPESQ) of the Universidade Federal do Rio Grande do Sul (UFRGS), Brazil, for a scholarship offered to Gustavo Flores Chapacais.
Funding
This study was supported by the Research Incentive Fund (FIPE/HCPA: grant no. 18-0246), Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico Universal (MCTI/CNPQ: grant no. 28/2018), and the Research Support Fund of the Sociedade de Reumatologia do Rio Grande do Sul.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karnopp, T.E., Chapacais, G.F., Freitas, E.C. et al. Lupus animal models and neuropsychiatric implications. Clin Rheumatol 40, 2535–2545 (2021). https://doi.org/10.1007/s10067-020-05493-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05493-7