Abstract
Introduction
This study aimed to characterize disease burden among patients with rheumatoid arthritis (RA) with moderate-to-high disease activity who had received conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) monotherapy for ≥ 6 months but had not advanced to a biologic therapy.
Methods
Patients enrolled in the US Corrona RA Registry between June 1, 2014, and January 30, 2018, with 6 months of continuous csDMARD monotherapy, with moderate-to-high disease activity, who remained biologic naive, and who had ≥ 1 follow-up visit were identified. Disease activity was assessed among patients with a 6-month follow-up visit (± 3 months). Descriptive statistics were used to compare demographics and disease characteristics between patients with or without treatment advancement.
Results
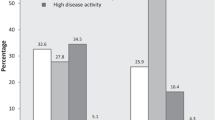
The study included 409 patients with a disease activity assessment at 6 months (mean (SD) age 65.9 (12.6) years; mean duration of csDMARD therapy 407 (221) days). Of those patients, more than half (54%, n = 219) remained in moderate-to-high disease activity. Patients remaining in moderate-to-high vs. remission-to-low disease activity had higher baseline swollen (6.1) and tender joint counts (6.8). Over the 6-month period, treatment advancement occurred in 29% of patients. Those who advanced treatment (n = 118) vs. did not advance treatment (n = 291) were younger, had a shorter duration of RA, had higher disease activity, and reported higher levels of pain and fatigue.
Conclusions
The substantial number of patients with persistent moderate-to-high disease on csDMARDs over a 6-month period and who did not advance treatment indicates that there is considerable need for a treat-to-target approach to care for patients with RA.
Key Points •For patients with RA and an inadequate response to treatment with initial csDMARD monotherapy, guidelines recommend treatment advancement; however, this may not be occurring in real-world clinical settings. •In the current study, a substantial proportion of patients (54%) on csDMARDs had persistent moderate-to-severe disease activity at the 6-month (± 3 months) follow-up visit; however, only 29% of patients had their medication treatment advanced, indicating that there is considerable need for a treat-to-target approach to care for patients with RA. •Patients with younger age, shorter RA duration, and higher disease activity were more likely to have their medication treatment advanced, which suggests that potentially more aggressive treatment of disease activity is needed across the whole RA population. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a complex inflammatory disease characterized by symmetric and erosive arthritis typically affecting small- and medium-sized joints [1, 2]. When not treated effectively, RA can cause significant pain and progressive joint damage that can lead to disability and a reduced quality of life [1, 2]. The disease burden in RA is associated with multiple comorbidities and psychosocial impairments [1, 2]. Conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) are recommended as the first-line treatment for patients with RA [3,4,5].
For patients who have active disease despite initial csDMARD monotherapy, guidelines recommend treatment advancement to a combination of csDMARDs and/or use of biologics or Janus kinase inhibitors (JAKi) [3,4,5]. In clinical practice, it is common for patients treated with csDMARDs to remain in a moderate-to-high disease state without treatment advancement to a biologic or targeted synthetic DMARD [6]. Although there is international consensus on treatment advancement until remission-to-low disease activity is achieved [7], it is unknown whether this occurs consistently in everyday clinical practice. Patients with RA who remain in a moderate-to-high disease activity state have worse short- and longer-term outcomes (increased pain burden, disability, irreversible joint damage, etc.) [8].
This study sought to characterize disease burden among patients with RA with moderate-to-high disease activity who received csDMARD monotherapy for at least 6 months but whose treatment had not been advanced to a biologic or JAKi. Among those with moderate-to-high disease activity, treatment patterns and disease activity were assessed at a subsequent visit 6 months later and compared between those patients who achieved remission-to-low disease activity and those who remained moderate-to-high at that 6-month visit; treatment patterns and patient characteristics were also compared between those who advanced treatment and those who did not. This study also aimed to identify factors associated with treatment advancement.
Materials and methods
Data source
This was a retrospective, descriptive analysis of the US Corrona RA registry. The US Corrona RA Registry is an independent, prospective, observational cohort of patients with RA that was initiated in 2001 [9]. As of December 31, 2018, the US Corrona RA Registry included data from about 50,600 patients with approximately 384,400 visits and approximately 180,600 person-years of follow-up. Information on disease duration, disease severity and activity, comorbidities, medication usage, and patient-reported outcome (PRO) data on clinical outcomes are collected from both patients and their treating rheumatologists.
All patients provided written informed consent and authorization before enrollment in the Corrona registry. Participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Institutional review board (IRB) approvals for the registry and the current study were obtained from a central IRB (New England IRB (NEIRB; No. 120160610)) for private practice sites and from local IRBs for each participating academic site.
Study population
Adult patients (aged ≥ 18 years) enrolled in the US Corrona RA Registry between June 1, 2014, and January 30, 2018, continuously receiving csDMARD monotherapy (hydroxychloroquine, leflunomide, methotrexate, or sulfasalazine) for at least 6 months were eligible for inclusion. Patients with prior biologic or JAKi exposure were excluded from the study. Eligible patients had moderate-to-high disease activity (Clinical Disease Activity Index (CDAI) > 10) at the index visit (baseline); patients with a CDAI ≤ 10 at the index visit were excluded. Patients were required to have had an index visit with at least one 6-month follow-up visit. The 6-month follow-up visit was defined as any visit within the 3–9 months window after the index visit/baseline period. The baseline period was defined as the 6-month continuous csDMARD monotherapy and biologic-naive treatment period prior to the index visit (Fig. 1).
Data analysis
An initial descriptive analysis was conducted to assess the proportion of patients with moderate-to-high disease activity despite continued 6-month csDMARD monotherapy. The total study population was further divided into two cohorts based on moderate-to-high (CDAI > 10) vs. remission-to-low (CDAI ≤ 10) CDAI level at the 6-month follow-up visit. The distributions of patient demographics, clinical characteristics, and disease activity at baseline were assessed in cohorts CDAI > 10 or CDAI ≤ 10 by descriptive statistics.
The changes from baseline to follow-up in disease activity measures were also assessed descriptively and compared between the two visits (baseline and 6-month follow-up visit) by pairwise t-test for continuous outcomes and McNemar’s test for paired nominal measures in total as well as within each cohort (CDAI > 10 or CDAI ≤ 10).
Among patients with a disease activity assessment at the 6-month follow-up visit, descriptive statistics were used to evaluate patient demographics, clinical characteristics, and disease activities at baseline among three groups of RA disease duration (< 2 years, 2–5 years, > 5 years). Statistical significance of the association between each of these variables and disease duration were determined using chi-square tests for categorical variables and the Kruskal-Wallis one-way analysis of variance (ANOVA) for continuous measures. The change in disease activity from baseline was also assessed descriptively and stratified by disease duration (< 2 years, 2–5 years, > 5 years). The Kruskal-Wallis one-way ANOVA was used to determine if there were any statistically significant differences between the mean changes of disease activity among the three groups of disease duration. Additionally, the baseline characteristics of those patients who advanced treatment vs. those who did not advance treatment and were persistent in a moderate-to-high disease activity state after 6 months was reported. Treatment advancement was defined as either a dose escalation of the initial csDMARD or initiation of a DMARD (conventional synthetic, targeted synthetic, and biologic) from the index visit to the 6-month visit. Patient characteristics between patients with or without therapy advancement were assessed by descriptive statistics and compared by Wilcoxon’s rank-sum test for continuous variables and chi-square test for categorical measures.
Exploratory analyses were used to determine factors associated with lack of advancement in patients who persisted with moderate-to-high disease activity. The frequency and percentage of patients who received each treatment advancement type were calculated. Univariate and multiple logistic regression models were utilized to predict the probability of patients’ advancement based on baseline demographic and clinical characteristics and to quantify how strongly each of these prognostic factors was associated with treatment advancement by odds ratio (OR).
Results
Overall study population
A total of 23,022 patients aged 18 years or older with RA had enrolled in the US Corrona RA Registry as of June 1, 2014. Of 2982 biologic-naïve patients on csDMARD monotherapy for at least 6 months, a total of 809 (27%) patients persisted with moderate-to-high disease activity and were considered for this study; 525/809 completed a visit at least 3 months after their index visit and were therefore eligible for inclusion. Of those, 414 completed a follow-up visit at 3–9 months after the index visit and 409 had a CDAI assessment at 6 months (Fig. 2). The mean (SD) age of patients with the CDAI assessment at 6 months was 65.9 (12.6), and patients had a mean duration of csDMARD therapy of 407 days (221). Further details on patient demographics are provided in Table 1.
Disease activity
Despite receiving csDMARDs continuously for ≥ 6 months prior to cohort entry, 54% (219/409) of patients with a CDAI assessment had persistent moderate-to-severe disease activity at the 6-month (± 3 months) follow-up visit.
Among patients with CDAI > 10 at 6 months (n = 219), no significant improvement in disease activity was observed (Table 2), but an increased mean modified Health Assessment Questionnaire (mHAQ) score (p = 0.040) at the 6-month follow-up visit was noted. Apart from achieving CDAI remission-to-low disease activity at the 6-month visit, there was also strong evidence that patients in the CDAI ≤ 10 at 6 months cohort experienced significant improvement in other outcomes, including pain (p < 0.001), fatigue (p < 0.001), morning stiffness duration (p = 0.01), and mHAQ (p = 0.03) (Table 2).
When stratified by disease duration, baseline characteristics such as age, sex, race, and comorbidities were similar across the three disease duration categories of < 2, 2–5, and ≥ 5 years in the group of patients with CDAI > 10 at 6 months (data not shown). Patients with a shorter disease duration (< 2 years) had significantly higher baseline disease activity (p = 0.004) and tender joint counts (p < 0.001) (data not shown). Among patients with CDAI ≤ 10 at 6 months stratified by disease duration, changes in disease activity from baseline to the 6-month follow-up visit were larger among patients with a shorter disease duration, and patients with an RA disease duration of < 2 years had a significantly larger decrease in tender joint count (p = 0.016) compared with patients with longer disease durations (Table 3).
Treatment advancement at 6 months
Of the patients who completed a visit at 3–9 months after the index date, treatment advancement occurred in 118 (29%) patients, with 291/409 (71%) having no change. Dose escalation of the csDMARD, initiation of another csDMARD, and initiation of a biologic DMARD occurred in 55 (13%), 33 (8%), and 40 (10%) patients in the total population (n = 409), respectively (data not shown). Patients who did not advance but still had CDAI > 10 after 6 months had a mean (SD) age 66.6 (12.4) years, duration of csDMARD use of 371.8 (202.3) days, and duration of RA of 11.4 (11.0) years.
Medication advancement was significantly associated with younger age (p = 0.007), shorter duration of RA disease (p < 0.001), use of prednisone at doses ≥ 10 mg (p = 0.023), lower EuroQol 5 Dimensions (EQ-5D) scores (p = 0.007), and higher disease activity measures of CDAI (p = 0.008), tender joint counts (p < 0.001), patient global (p = 0.021), patient pain (p = 0.009), patient-reported fatigue (p = 0.002), modified Disease Activity Scale 28 (mDAS28) (p < 0.001), mHAQ (p = 0.035), and duration of morning stiffness (p = 0.012) at baseline (Table 4). Among patients with medication advancement, significantly greater changes in disease activity measures at the 6-month follow-up visit relative to baseline were observed for patient global (p = 0.006), patient pain (p = 0.021), patient-reported fatigue (p = 0.027), and duration of morning stiffness (p = 0.029).
The results from univariate logistic regression analysis further confirmed the relationship of patient baseline characteristics with medication advancement, with the exception of mHAQ. The significant variables from the univariate analysis served as candidate variables for multiple logistic regression analysis. Multiple logistic regression analysis results showed that medication advancement was significantly associated with RA duration (OR = 0.97, p = 0.032), even after adjusting for nonsteroidal anti-inflammatory drug use (OR = 1.31, p = 0.26), high CDAI score (OR = 1.02, p = 0.15), and patient fatigue (OR = 1.01, p = 0.15).
Discussion
csDMARDs are recommended as the first-line treatment for patients with RA. However, for patients who have active disease despite csDMARD monotherapy, guidelines recommend treatment advancement to a combination of csDMARDs and/or use of biologics [3,4,5]. More than 50% of patients with RA treated with csDMARD monotherapy in our study did not achieve remission-to-low disease activity. Despite a substantial number of patients with persistent moderate-to-high disease activity and guideline recommendations to advance treatment in such cases, treatment advancement was observed in < 30% of patients. This finding is in agreement with a recent study looking at prescribing patterns of biologic and nonbiologic DMARDs among patients with RA, which reported that a large number of patients did not receive care consistent with guideline recommendations [10].
In the present study, more than half of patients with RA did not have an adequate response to csDMARD monotherapy and a significant increase in mean mHAQ was observed at the 6-month visit. According to historical studies, HAQ scores are typically high at disease onset and then increase slowly over time (0.01–0.016 units per year) [11]. Additionally, in patients with RA, a change in mHAQ of 0.25 has been suggested to be clinically meaningful [12]. A significant increase in mHAQ from 0.49 to 0.54 (p = 0.04) over a 6-month period may indicate more rapidly progressing and clinically significant disability in patients who continued with uncontrolled disease. Furthermore, a substantial number of patients on csDMARDs (54% of 409 patients) had persistent moderate-to-high disease activity over 6 months of follow-up. These patients who persisted with moderate-to-high disease activity and did not advance treatment had a mean RA duration of 11.4 (10.99) years. Patients who did advance therapy tended to be younger, had a shorter duration of RA, had higher disease activity at baseline, and had a significantly higher baseline mean mHAQ.
In addition to elucidating the proportion of patients who do not advance therapy despite persistent moderate-to-high disease activity over 6 months of follow-up, it is also important to understand the factors that may be associated with the likelihood of therapy advancement. In this study, patients with younger age, shorter RA duration, and higher disease activity were more likely to have their medication treatment advanced leading to improvements in patient-reported pain, fatigue, and morning stiffness duration among these patients. Conversely, 54% of patients remained in a moderate-to-high disease activity state and did not experience improvements in disease activity measures, which suggests that potentially more aggressive treatment is needed across the entire population of patients with RA. Consistent with the findings in this study, patient factors, such as disease duration, disease activity, and work status, have been described elsewhere as contributing to patients receiving care in line with treatment recommendations [13]. Additionally, treatment approaches have been determined to be less consistent with the published American College of Rheumatology recommendations in those with moderate disease activity and poor prognosis [10].
The majority of patients in the current study are not being managed with a treat-to-target philosophy despite its value being widely accepted and despite advanced therapies being readily available to treat patients after an inadequate response to csDMARD monotherapy. Several challenges to implementing the treat-to-target approach to care exist, including the design of clinical medicine, patient reluctance to take therapies, and economic factors [13,14,15,16,17,18]. A lack of response to persistent active disease, defined as “clinical inertia,” has been documented for other chronic illnesses [14]. Furthermore, many healthcare providers are not designed or trained to routinely assess disease activity, and many practitioners are not engaging in shared decision-making with patients regarding accelerating medications [16]. Patient hesitation may be another factor in the lack of dose escalation [18]. A higher prevalence of skipping medications owing to limited economic means has also been reported for patients with RA compared with patients with other chronic illnesses [15]. A risk/benefit analysis study determined that patients are willing to take on significant risk to achieve benefits from RA medications, such as reductions in pain and swollen joints, especially patients with severe disease [17]. Physicians should take into consideration patient preferences, quality of life, economic factors, and willingness to take on an increased risk of adverse events when discussing treatment plans with patients with RA.
Despite the guidelines recommending treatment advancement when there is an inadequate response to initial csDMARD monotherapy [3, 4], this may not be occurring in real-world clinical settings. It is appropriate to re-evaluate treatment after 3–6 months, and patients who are not responding to treatment should have their dose escalated, switch therapy, or have an additional therapy added to their current regimen [3, 4]. Systematically evaluating disease activity and responding with titrating medications is something healthcare providers should consider for patients with RA. Additionally, understanding of the disease burden among patients with RA on csDMARD treatment could lead to the development of new treatment regimens for patients.
Limitations
This study has several limitations. As with any registry study, it is hard to assess whether the patients are representative of the total population of patients with RA across the USA in terms of clinical characteristics and RA treatment practices given there is no national health plan that enrolls all patients; however, a recent study has shown that Medicare beneficiaries enrolled in the US Corrona Registry share similar demographic and clinical characteristics with Medicare beneficiaries with claims for RA who were not enrolled [19]. Although this study did not evaluate whether financial concerns may have been a factor in treatment decisions, it did examine treatment patterns for at least 3 months (up to 9 months) after the index visit; however, it may take more time for providers to incorporate therapy advancements and treat-to-target recommendations in clinical practice. It may be appropriate to delay treatment advancement if patients have borderline elevated disease activity or elevated disease activity due to a time-limited event. It is also unknown whether patients were offered therapies but declined to advance their treatment; this was shown to occur in a recently published treat-to-target trial which documented reasons for patient unwillingness to advance treatment [18]. Additionally, this study did not systematically identify comorbidities which can contribute to the clinical decision to not advance treatment. Finally, because of less frequent reports of visits and/or prior or current biologic use, some of the patients enrolled in the US Corrona RA Registry did not meet the inclusion criteria for this study.
Conclusion
As described in this article, a substantial number of patients with RA continue to be managed with csDMARD monotherapy despite remaining in moderate-to-high disease activity at a 6-month follow-up visit. This indicates that there is considerable need for a treat-to-target approach to care to prevent joint damage and physical disability and maximize long-term health-related quality of life for patients with RA. Furthermore, future studies are warranted to elucidate factors related to the response variation of csDMARD and treatment selection for RA disease.
References
Kvien TK (2004) Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 22(2 Suppl 1):1–12. https://doi.org/10.2165/00019053-200422001-00002
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4
NICE (2018) Rheumatoid arthritis in adults: management. http://nice.org.uk/guidance/ng100. Accessed 02/18/2019
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T, American College of R (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 68(1):1–25. https://doi.org/10.1002/acr.22783
Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, Bykerk V, Dougados M, Emery P, Haraoui B, Gomez-Reino J, Kvien TK, Nash P, Navarro-Compan V, Scholte-Voshaar M, van Vollenhoven R, van der Heijde D, Stamm TA (2016) Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 75(1):16–22. https://doi.org/10.1136/annrheumdis-2015-207526
Wei W, Knapp K, Wang L, Chen CI, Craig GL, Ferguson K, Schwartzman S (2017) Treatment persistence and clinical outcomes of tumor necrosis factor inhibitor cycling or switching to a new mechanism of action therapy: real-world observational study of rheumatoid arthritis patients in the United States with prior tumor necrosis factor inhibitor therapy. Adv Ther 34(8):1936–1952. https://doi.org/10.1007/s12325-017-0578-8
Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, Kvien TK, Navarro-Compan MV, Oliver S, Schoels M, Scholte-Voshaar M, Stamm T, Stoffer M, Takeuchi T, Aletaha D, Andreu JL, Aringer M, Bergman M, Betteridge N, Bijlsma H, Burkhardt H, Cardiel M, Combe B, Durez P, Fonseca JE, Gibofsky A, Gomez-Reino JJ, Graninger W, Hannonen P, Haraoui B, Kouloumas M, Landewe R, Martin-Mola E, Nash P, Ostergaard M, Ostor A, Richards P, Sokka-Isler T, Thorne C, Tzioufas AG, van Vollenhoven R, de Wit M, van der Heijde D (2016) Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 75(1):3–15. https://doi.org/10.1136/annrheumdis-2015-207524
Radner H, Smolen JS, Aletaha D (2014) Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther 16(1):R56. https://doi.org/10.1186/ar4491
Kremer JM (2005) The CORRONA database. Clin Exp Rheumatol 23(5 Suppl 39):S172–S177
Harrold LR, Harrington JT, Curtis JR, Furst DE, Bentley MJ, Shan Y, Reed G, Kremer J, Greenberg JD (2012) Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum 64(3):630–638. https://doi.org/10.1002/art.33380
Maska L, Anderson J, Michaud K (2011) Measures of functional status and quality of life in rheumatoid arthritis: health assessment questionnaire disability index (HAQ), modified health assessment questionnaire (MHAQ), multidimensional health assessment questionnaire (MDHAQ), health assessment questionnaire II (HAQ-II), improved health assessment questionnaire (improved HAQ), and rheumatoid arthritis quality of life (RAQoL). Arthritis Care Res 63(Suppl 11):S4–S13. https://doi.org/10.1002/acr.20620
Wolfe F, Pincus T (1999) Listening to the patient: a practical guide to self-report questionnaires in clinical care. Arthritis Rheum 42(9):1797–1808. https://doi.org/10.1002/1529-0131(199909)42:9<1797::AID-ANR2>3.0.CO;2-Q
Harrold LR, Reed GW, Kremer JM, Curtis JR, Solomon DH, Hochberg MC, Kavanaugh A, Saunders KC, Shan Y, Spruill TM, Pappas DA, Greenberg JD (2016) Identifying factors associated with concordance with the American College of Rheumatology rheumatoid arthritis treatment recommendations. Arthritis Res Ther 18:94. https://doi.org/10.1186/s13075-016-0992-3
Phillips LS, Twombly JG (2008) It’s time to overcome clinical inertia. Ann Intern Med 148(10):783–785
Harrold LR, Briesacher BA, Peterson D, Beard A, Madden J, Zhang F, Gurwitz JH, Soumerai SB (2013) Cost-related medication nonadherence in older patients with rheumatoid arthritis. J Rheumatol 40(2):137–143. https://doi.org/10.3899/jrheum.120441
Harrold LR, Reed GW, Harrington JT, Barr CJ, Saunders KC, Gibofsky A, Greenberg JD, John A, Devenport J, Kremer JM (2014) The rheumatoid arthritis treat-to-target trial: a cluster randomized trial within the Corrona rheumatology network. BMC Musculoskelet Disord 15:389. https://doi.org/10.1186/1471-2474-15-389
Husni ME, Betts KA, Griffith J, Song Y, Ganguli A (2017) Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int 37(9):1423–1434. https://doi.org/10.1007/s00296-017-3760-z
Harrold LR, Reed GW, John A, Barr CJ, Soe K, Magner R, Saunders KC, Ruderman EM, Haselkorn T, Greenberg JD, Gibofsky A, Harrington JT, Kremer JM (2018) Cluster-randomized trial of a behavioral intervention to incorporate a treat-to-target approach to care of US patients with rheumatoid arthritis. Arthritis Care Res 70(3):379–387. https://doi.org/10.1002/acr.23294
Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, Kremer J, Setoguchi S, Solomon DH, Xie F, Yun H (2014) Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res 66(12):1790–1798. https://doi.org/10.1002/acr.22377
Funding
This study is sponsored by Corrona, LLC. Access to study data was limited to Corrona and Corrona statisticians completed all of the analysis; all authors contributed to the interpretation of the results. Corrona, LLC has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Crescendo, Eli Lilly and Company, Genentech, Gilead, GSK, Janssen, Merck, Momenta Pharmaceuticals, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Roche, Sun, UCB, and Valeant. Financial support for the study was provided by AbbVie. AbbVie participated in the design of the study, study conduct, interpretation of data, review, and approval of the manuscript.
Medical writing support was provided by Brandy Menges, PhD, and Fiona Woodward, PhD, CMPP, of JK Associates Inc., a member of the Fishawack Group of Companies; this support was funded by AbbVie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients provided written informed consent and authorization before enrollment in the Corrona registry. Participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Institutional review board (IRB) approvals for the registry and the current study were obtained from a central IRB (New England IRB (NEIRB; No. 120160610)) for private practice sites and from local IRBs for each participating academic site. For academic sites that did not receive a waiver to use the central IRB, documentation of approval from the respective local IRB was submitted to Corrona, LLC, prior to the initiation of any study procedures.
Conflict of interest
LR Harrold is an employee and shareholder of Corrona, LLC. She has been a consultant to AbbVie, BMS, and Roche and had a research grant from Pfizer.
HJ Litman and H Feng are employees of Corrona, LLC.
PA Patel, J Griffith, and CA Schlacher are employees of AbbVie and own AbbVie stock.
JM Kremer is an employee and shareholder of Corrona, LLC; a consultant for AbbVie, Amgen, BMS, Genentech, Lilly, Regeneron, Sanofi, and Pfizer and received research grants from: AbbVie, Genentech, Lilly, Novartis, and Pfizer.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harrold, L.R., Patel, P.A., Griffith, J. et al. Assessing disease severity in bio-naïve patients with RA on treatment with csDMARDs: insights from the Corrona Registry. Clin Rheumatol 39, 391–400 (2020). https://doi.org/10.1007/s10067-019-04727-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04727-7