Abstract
According to international recommendations, the selection of the biologic disease modifying anti-rheumatic drug (bDMARD) for rheumatoid arthritis (RA) is mainly left to the clinician’s preference. We analyzed the real-life factors influencing the first-line choice or the switching strategy, focusing on the prescription of abatacept (ABA) or tocilizumab (TCZ) compared to TNFα inhibitors (TNFi). Patients enrolled in the Lombardy Rheumatology Network (LORHEN) Registry after January 1, 2010, when all considered bDMARD agents were available, were included. The population was divided into “first-” and “second-line” bDMARD. We included 1910 patients (first line n = 1264, second line n = 646). Age was higher in ABA or TCZ vs TNFi treated patients (p < 0.0001). Positive latent tuberculosis screening was associated with first-line ABA (p = 0.002). Methotrexate (MTX) combination therapy was lower in the TCZ group (p = 0.02). The type (dyslipidemia, hypertension, pulmonary disease) and the number of comorbidities influenced the choice towards ABA (p = 0.01). Multinomial logistic regression demonstrated that a second-line treatment, higher age, dyslipidemia, pulmonary disease, other comorbidities, and extra-articular RA manifestations were associated with ABA compared to TNFi. TCZ was associated with a second-line treatment, higher age, and more severe disease activity. Stopping the first bDMARD due to adverse events (AE) influenced the choice towards ABA. In real life, higher age and comorbidities influence the choice towards ABA and TCZ compared to TNFi. ABA was preferred in case of suspension of previous treatments due to AE. After failing a first-line TNFi, swapping to a different mechanism of action is more common.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, major advances have been brought to the management of rheumatoid arthritis (RA). Biologic disease modifying anti-rheumatic drugs (bDMARDs) have become the standard of care for the treatment of RA not adequately responding to conventional synthetic DMARDs (csDMARDs). To date, several bDMARDs acting at different levels of the immune response have been licensed for the treatment of RA. TNFα inhibitors (TNFis) encompass five different agents: infliximab (IFX), including the recently approved infliximab bio-similar (bs-IFX); etanercept (ETA), adalimumab (ADA); golimumab (GOL); and certolizumab pegol (CZP) [1], allowing to choose among different routes and frequency of administration and peculiar pharmacokinetic characteristics. The introduction of the interleukin-6 (IL-6) receptor blocking monoclonal antibody tocilizumab (TCZ), the T-cell co-stimulation inhibitor abatacept (ABA), and the anti-CD20 B-cell depleting agent rituximab (RTX) have further increased the therapeutic armamentarium to treat RA.
However, despite the wide range and evolving spectrum of bDMARD options available, little is still known on the best approach to the individual patient, and the choice of the first line or sequencing bDMARDs is still largely left to the clinician’s choice and personal experience.
Indeed, randomized controlled trials (RCTs), indirect comparison studies, meta-analysis, and head to head studies have failed to demonstrate a significant difference among the different classes of bDMARDs in terms of efficacy on clinical, functional, and radiographic outcomes [2–5]. The only exception being probably represented by TCZ monotherapy [6]. International recommendations [7] do not provide a preference on the mechanism of action (MoA) to be chosen as first bDMARD therapy. TNFis, ABA or TCZ, and, under certain circumstances such as history of lymphoma or demyelinating disease, RTX are recommended as first-line biologic agents. Switching among bDMARDs is also mainly left to the clinician’s decision between a second TNFi or a different MoA [6, 7]. However, it is generally accepted that switching from a second to a third TNFi is associated with significantly lower response to treatment and a different MoA should be considered in these patients [8]. Only scant and cautious acknowledgement of potential differences in the safety profile of available bDMARDs comes from the 2015 American College of Rheumatology (ACR) guideline for the treatment of RA [9] that indicates ABA, noteworthy with a very low level of evidence, as the drug of choice in case of previous serious infections and ABA or TCZ over TNFis in patients with a previous lymphoproliferative disorder.
Nevertheless, real-life data have emphasized prescription differences among bDMARDs, possibly influenced by the emerging evidence demonstrating a better safety profile of ABA [10, 11], and the unique efficacy of TCZ used as monotherapy, compared to TNFis. Moreover, in clinical practice, several other factors may be advocated as drivers of the choice of a specific agent such as comorbidities, host-related risk factors for infections, cardiovascular risk, the patient’s compliance and preference for a specific route of administration, predictive biomarkers such as seropositivity for rheumatoid factor (RF) and/or anti-citrullinated protein antibodies (ACPA), and, eventually, also cost-effectiveness [12]. These factors are not always adequately supported by evidence-based medicine (EBM) but are perceived as very relevant by experts in the field and supported by science-based medicine (SBM).
The aim of this study was to retrospectively analyze the factors influencing the choice of the first-line bDMARD or the switching strategy in a large cohort of real-life RA patients enrolled in the Italian Lombardy Rheumatology Network (LORHEN) Registry, focusing on the prescription of ABA or TCZ compared to TNFis.
Materials and methods
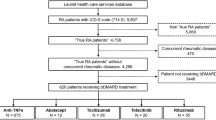
Patients enrolled in the Lombardy Rheumatology Network (LORHEN) Registry [13] including patients treated with bDMARDs in eight rheumatologic centers in Northern Italy were analyzed. The analysis was limited to patients enrolled after January 1, 2010, when all three different MoA were available. All patients provided written informed consent. The study population was divided into “first-line” and “second-line” bDMARD. Comorbidities were categorized into organ systems groups (pulmonary disease, cardiovascular disease, arterial hypertension, dyslipidemia, diabetes mellitus, peripheral neuropathy, osteoporosis, thyroid autoimmune disease, other comorbidities not belonging to the previous categories).
Statistical analysis
Analysis was performed using Stata 14.1 (StataCorp, College Station, TX, USA). A two-sided p value was considered statistically significant. Continuous data were described as mean and standard deviation (SD) or median and quartiles (IQR) and compared with the one-way ANOVA or the Kruskall-Wallis test. Categorical data were summarized as counts and percent and compared with the Fisher exact test. For pairwise post hoc comparison of ABA and TCZ vs TNFis, significance was set at 0.025 (Bonferroni correction). Multinomial logistic regression was used to assess the probability of using either treatments given line of treatment and adjusted for baseline characteristics and comorbidities. Confounders with p < 0.05 at univariate analysis together with extra-articular manifestations (of clinical interest) were included in the model in addition to line. The relative risk ratios (RRR) of choosing ABA rather than TNFi, or TCZ rather than TNFi, were reported together with 95% confidence intervals. Identification of predictors of the choice of treatment was also performed in a separate pre-specified subgroup analysis by line of treatment. Forrest plots were used to display results.
Results
First-line bDMARD treatment
A total of 1910 patients were included. Patients treated with a first-line bDMARD were 1264 (ABA first line: 115 patients; TCZ first line: 130; TNFi first line: 1019). Second-line bDMARD treatment included 646 patients (ABA second line: 143; TCZ second line: 97; TNFi second line: 406). General characteristics of the study population are displayed in Table 1. Mean age at the time of first bDMARD initiation was statistically different for ABA vs TNFi (58.74 ± 13.39 vs 53.42 ± 13.66; p = 0.0003) and for TCZ vs TNFi (57.84 ± 10.89 vs 53.42 ± 13.66; p = 0.002).
ABA was associated with a higher prevalence of positive LTBI status (29.63%) compared to TCZ (16.26%); p = 0.04, and TNFi (15.64%); p = 0.003. There were no significant differences among the three groups regarding the pre-treatment screening for HBV or HCV infections.
Data regarding treatment preceding the initiation of the first-line bDMARD and the current therapeutic characteristics are presented in Table 2. TCZ was significantly more prescribed as monotherapy compared to ABA (p = 0.02) and TNFi (p = 0.01).
Comorbidities and first-line bDMARD
The prevalence of comorbidities according to the organ system involved is shown in Table 3 Dyslipidemia was recorded in 14.78% of patients treated with ABA, whereas 10% (p = 0.99) of those treated with TCZ and 7.16% (p = 0.03) of patients started on TNFi were dyslipidemic. The comparison between TCZ and TNFi was not significant (p = 0.86). Arterial hypertension was more represented in ABA group compared to TNFi (p = 0.003).
Pulmonary comorbidities were statistically more represented in the ABA group compared to the TNFi group (p < 0.0001) and, not reaching statistical significance, the TCZ group (p = 0.07). The comparative frequency of pulmonary comorbidities between TCZ and TNFi was also performed (p = 0.57).
The prevalence of comorbidities categorized as “other” in the LORHEN Registry were reported to affect 67.83% of patients in the ABA group, 47.69% in the TCZ group (p = 0.006), and 41.51% of the TNFi group (p = <0.0001).
Pulmonary extra-articular involvement of disease was reported in 4 (3.48%) patients in the ABA treated group, 4 (3.08%) of the TCZ group, and 18 (1.77%) of the TNFi group; p = 0.25. Rheumatoid vasculitis was recorded in only 1 patient in the TNFi group. Rheumatoid nodules were reported for 6 (5.22%) patients treated with first-line ABA, 1 (0.77%) in the TCZ group, and 25 (2.45%) in the TNFi group; p = 0.09. Ocular involvement was reported in 6 (0.59%) patients treated with TNFi and none of the patients in the two other therapeutic groups (p = 1.0)
Having ≥two comorbidities was significantly associated with the prescription of ABA compared to both TCZ (p = 0.01) and TNFi (p = 0.02); Fig. 1.
Second-line bDMARD treatment
The general characteristics of the study population at the time of treatment switch to a second-line bDMARD are shown in Table 1.
The interruption of the first-line bDMARD due to adverse events (AE) influenced the choice of the second-line treatment in favor of ABA compared to TNFi: relative risk ratio (RRR): 3.37 (CI 1.28–8.83), Fig. 3, panel b. Neither ABA nor TCZ choice as second-line drugs was influenced by the discontinuation of the previous bDMARD due to primary or secondary inefficacy.
Age was significantly higher in the ABA second-line group compared to TNFi (p = 0.008) but not compared to TCZ (p = 0.20). Age difference between TCZ and TNFi was not significant (p = 0.84).
Disease activity at the time of switch was significantly higher when comparing ABA vs TNFi (p = 0.03) and TCZ vs TNFi (p < 0.0001). The difference in DAS28 between ABA and TCZ was not significant. Combination therapy with MTX with a second-line bDMARD was prescribed in 54.64% of patients treated with TCZ compared to 67.98% of patients in the TNFi group (p = 0.02) and 64.34% of patients on ABA (p = 0.08). The difference of concomitant MTX rates between ABA and TNFi was not significant (p = 0.47).
Comorbidities and second-line bDMARD
The prevalence of comorbidities at the time of switch to a second-line bDMARD is presented in Table 3. Peripheral neuropathy was significantly less represented in the TNFi group compared to ABA (p = 0.006).
Extra-articular manifestations of disease were not significantly more represented in any therapeutic group in the second-line bDMARD population.
Multinomial logistic regression analysis, exploring the role of treatment line adjusted for comorbidities and baseline characteristics on the choice of bDMARD, revealed that the choice of ABA compared to TNFi was significantly influenced by a second-line of treatment, age, extra-articular vasculitic manifestations of disease, dyslipidemia, pulmonary comorbidities, neuropathies, and other comorbidities in general. When analyzing the drivers of choice for TCZ, compared to TNFi, the following factors resulted significant: second line of treatment, age, and DAS28 disease activity.
The relative risk of choosing ABA compared to TCZ on a second-line bDMARD treatment was not significant.
The subanalysis of the factors influencing the choice of the bDMARD according to the different lines of treatment revealed that the choice of first-line ABA was influenced by age, a positive LTBI screening, extra-articular ocular and vasculitic involvement, arterial hypertension, pulmonary comorbidities, and other comorbidities in general. First-line TCZ choice was influenced by: age, extra-articular ocular and vasculitic involvement, diabetes, and neuropathies.
Regarding second-line treatment, ABA second-line was mainly selected according to the following drivers: age, suspension of first-line bDMARD due to AE, extra-articular nodulosis, and neuropathy. On the other hand, high DAS28 disease activity, positive LTB screening, pulmonary or cardiovascular comorbidities, and neuropathy influenced the choice of second-line TCZ vs TNFi. The Forrest plots describing the drivers of choice of ABA and TCZ vs TNFi are represented in Figs. 2 and 3.
Discussion
The introduction of bDMARDs to the standard of care for RA has revolutionized the course of the disease. Despite the constantly developing therapeutic options and an increasing shift of interest towards the management of withdrawing bDMARDs when remission is achieved [14–16], robust evidence is still needed on the appropriate prescription strategy of first-line bDMARD and switching strategy in real-life settings. To the best of our knowledge, this is the first study demonstrating that higher age and comorbidities influence the choice towards ABA and TCZ compared to TNFi in a large registry study. The interruption of previous bDMARD treatments due to AE also drove the choice towards ABA. Moreover, despite lack of a definite recommended strategy coming from international guidelines, a tendency of swapping to a different MoA after failing a first bDMARD seemed to be the preferred approach in real life.
The management of elderly patients with RA is deemed to increase in the future. Previous studies have shown that increasing age is associated with reduced chances of receiving TNFi compared to younger patients, despite higher disease activity levels [17, 18]. Moreover, age and comorbidities have been associated with a decreased response to ETA treatment [19, 20]. A recent study analyzing the comparative effects of bDMARDs on cardiovascular risk among more than 47,000 older patients with RA reported a higher risk of acute myocardial infarction with TNFi (particularly ETA and IFX) compared to ABA or TCZ [21]. Nevertheless, the influence of comorbidities on disease activity and potentially on treatment choices is not confined to older patients with RA [22]. In the first study reporting the influence of comorbidities on disease course in RA, about 27% of patients with early RA had already at least one chronic coexisting disease, the most frequently reported being cardiovascular (29%), respiratory (18%), and dermatological (11%) conditions [23].
In 2016, Innala et al. [24] reported an even higher prevalence of comorbidities, as high as 53.2%, in early RA patients. The commonest being hypertension (27.3%), obstructive pulmonary disease (13.9%), diabetes (8.0%), hypothyroidism (6.3%), and malignancy (5.0%). Interestingly, after 5 years, up to 41% of patients developed at least one new comorbidity, mainly cardiovascular, neoplastic, or osteoporosis. The high prevalence of comorbid conditions in patients with RA has been confirmed by a number of studies, among which, the COMOrbidities in Rheumatoid Arthritis (COMORA) study [25], a large international, cross-sectional study recruiting 4586 patients, highlighted the high prevalence of comorbidities and related risk factors in patients with RA and the need for an optimization of treatment.
The prevalence of comorbidities in our study was generally in line with previous epidemiologic reports [25]; however, we demonstrated that the number and the type of comorbidity differ among the three different therapeutic agents and may influence the choice of bDMARD treatment. First-line biologic agent was preferably ABA vs TCZ or TNFi in patients with concomitant comorbidities such as dyslipidemia or hypertension. The presence of a concomitant pulmonary condition was associated with a relative risk (RR) of 2.19 (95% CI: 1.05–2.4) of choosing ABA compared to TNFi. Unfortunately, the nature of this registry study does not allow to explore this finding in deeper detail, but it may be speculated that interstitial lung disease (ILD) might represent the main underlying pulmonary comorbidities leading to the choice of ABA over TNFi [26, 27]. Another possible explanation could regard the infectious risk. Our study demonstrated that in real life, ABA represents the drug of choice in case of underlying infectious risk, such as pre-treatment LTBI positive screening. A recent Italian multidisciplinary expert panel named Tailored BIOlogic therapy (ITABIO), aiming at defining an evidence-based decisional statements for the first-line-tailored biologic therapy in patients with rheumatic diseases, also concluded that LTBI positivity should drive the choice towards ABA, TCZ, or ETA [12]. Knowing the pathophysiologic role of TNF in controlling and confining mycobacteria infections [28], the choice of a different MoA whenever possible might not seem unexpected. However, safety data regarding ABA demonstrated that this agent is particularly associated with a lower incidence of serious infections (SI), in general, compared to other bDMARDs [10, 29]. The AMPLE trial [2], a head to head comparison of ABA vs ADA demonstrated that overall AE and serious infections were significantly lower with ABA: SI rate of 3.8 compared to 5.8% in the ADA-treated group. Also, the ATTEST trial reported considerably lower rates of SI in patients treated with ABA (1.9%) vs IFX (8.5%) [30]. A meta-analysis by Salliot et al. confirmed that ABA did not significantly increase the risk of SI in RA patients [31]. Our study confirmed the real-life strategy of preferring ABA in case of interruption of previous bDMARDs due to AE.
The significantly higher prevalence of monotherapy bDMARD prescription in the TCZ group confirmed by our study reflects the available evidence supporting the comparable efficacy of TCZ when prescribed with or without concomitant MTX [6, 7, 32, 33]. However, a critical look at this approach was risen by Gabay et al. [34] recently reporting that despite comparable clinical response, TCZ retention was shorter when prescribed as monotherapy. The Italian real-life switching strategy also suggests that swapping, compared to cycling of bDMARDs, often represents the preferred approach after failing a first-line bDMARD. ABA was associated with a significant relative risk (RR) of 3.2 times (95% CI 1.71–6.02) of being chosen as second-line treatment compared to TNFi, while TCZ showed a RRR of 2.01 (95% CI 1.2–3.36) vs TNFi. This practice has been previously demonstrated to lead to longer drug survival, irrespective of the reason for discontinuing the first TNFi [35]. In our study, second-line TCZ was also associated with higher disease activity compared to patients treated with TNFi. Lee et al. [36] recently analyzed the comparative efficacy and safety of different therapeutic strategies available for RA patients not adequately responding to TNFi and demonstrated that TCZ was the second-line non-TNF bDMARD with the highest performance regarding an early good response and acceptable safety profile.
Our study has some limitations. The retrospective nature of the analysis may be prone to assignment for treatment and patient selection bias. Given the registry nature of the data collection, some detailed information might be missing and comorbidity classification (i.e., osteoporosis or autoimmune thyroid disease, other comorbidities) may have not been fully useful for the purposes of our study of identifying the comorbidities potentially influencing the treatment strategy. Nevertheless, these information added a valuable picture on the general prevalence of comorbidities in a large cohort of patients treated with biological agents. Another pitfall of our study is the relatively high percentage of missing data on ACPA and/or RF positivity that could not therefore be used to accurately analyze their potential role in the biologic drug choice. Moreover, we decided not to include Rituximab in the comparison given the relatively smaller size of the population treated with this agent, the peculiar and well-known potential drivers of choice associated with this agent, and the different frequency of administration and maintenance regimen compared to other bDMARDs. We could not evaluate the influence of costs or the patient’s preferences for a specific route of administration in guiding the therapeutic decisions, as this aspect could not be gathered from the design of the registry.
Conclusions
In conclusion, despite lack of shared consensus and clear indications by international recommendations on the most adequate prescribing approach of bDMARD in patients with RA, our real-life study demonstrated that age, infectious risk, the number and type of comorbidities, and monotherapy are the main factors influencing the choice of the biologic drug in real life, driving the choice towards ABA or TCZ compared to TNFi. The interruption of previous bDMARDs due to AE influenced the choice towards ABA. After failing a first-line TNFi, a strategy of swapping to a different MoA is usually more common.
References
Nam JL, Ramiro S, Gaujoux-Viala C et al (2014) Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 73(3):516–528
Schiff M, Weinblatt ME, Valente R et al (2014) Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 73:86–94
Pierreisnard A, Issa N, Barnetche T, Richez C, Schaeverbeke T (2013) Meta-analysis of clinical and radiological efficacy of biologics in rheumatoid arthritis patients naive or inadequately responsive to methotrexate. Jt Bone Spine Rev Rhum 80:386–392
Favalli EG, Bugatti S, Biggioggero M, Caporali R (2014) Treatment comparison in rheumatoid arthritis: head-to-head trials and innovative study designs. Biomed Res Int 2014:831603
Jobanputra P, Maggs F, Deeming A, et al. (2012) A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open 2(6)
Gabay C, Emery P, van Vollenhoven R et al (2013) Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381(9877):1541–1550
Smolen JS, Landewé R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Caporali R, Sarzi-Puttini P, Atzeni F et al (2010) Switching TNF-alpha antagonists in rheumatoid arthritis: the experience of the LORHEN registry. Autoimmun Rev 9:465–469
Singh JA, Saag KG, Bridges SL, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol
Tanaka Y, Kubo S, Yamanaka H et al (2014) Efficacy and safety of abatacept in routine care of patients with rheumatoid arthritis: Orencia® as Biological Intensive Treatment for RA (ORBIT) study. Mod Rheumatol 24:754–762
Yun H, Xie F, Delzell E, Levitan EB et al (2016) Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in Medicare. Arthritis Rheumatol Hoboken NJ 68:56–66
Cantini F, Niccoli L, Nannini C, et al. (2015) Tailored first-line biologic therapy in patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum
Sarzi-Puttini P, Antivalle M, Marchesoni A et al (2008) Efficacy and safety of anti-TNF agents in the Lombardy rheumatoid arthritis network (LORHEN). Reumatismo 60:290–295
van den Broek M, Lems WF, Allaart CF (2012) Do we need guidelines to stop as well as to start biological therapies for rheumatoid arthritis? Clin Exp Rheumatol 30(4 Suppl 73):S21–S26
Tanaka Y, Hirata S (2013) Is it possible to withdraw biologics from therapy in rheumatoid arthritis? Clin Ther 35:2028–2035
Moghadam MG, Vonkeman HE, Ten Klooster PM, et al. (2016) Stopping tumor necrosis factor-inhibitors in patients with established rheumatoid arthritis in remission or stable low disease activity: a pragmatic randomized multicenter open-label controlled trial. Arthritis Rheumatol Hoboken NJ
Radovits BJ, Fransen J, Eijsbouts A, van Riel PLCM, Laan RFJM (2009) Missed opportunities in the treatment of elderly patients with rheumatoid arthritis. Rheumatology 48:906–910
Morsley K, Kilner T, Steuer A (2015) Biologics prescribing for rheumatoid arthritis in older patients: a single-center retrospective cross-sectional study. Rheumatol Ther 2:165–172
Martin WJ, Shim M, Paulus HE et al (2014) Older age at rheumatoid arthritis onset and comorbidities correlate with less health assessment questionnaire-disability index and clinical disease activity index response to etanercept in the RADIUS 2 registry. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis 20:301–305
Sugihara T, Harigai M (2016) Targeting low disease activity in elderly-onset rheumatoid arthritis: current and future roles of biological disease-modifying antirheumatic drugs. Drugs Aging 33:97–107
Zhang J, Xie F, Yun H, et al. (2016) Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis
Crepaldi G, Scirè CA, Carrara G et al (2016) Cardiovascular comorbidities relate more than others with disease activity in rheumatoid arthritis. PLoS One 11:e0146991
Kroot EJ, van Gestel AM, Swinkels HL, Albers MM, van de Putte LB, van Riel PL (2001) Chronic comorbidity in patients with early rheumatoid arthritis: a descriptive study. J Rheumatol 28:1511–1517
Innala L, Sjöberg C, Möller B et al (2016) Co-morbidity in patients with early rheumatoid arthritis - inflammation matters. Arthritis Res Ther 18:33
Dougados M, Soubrier M, Antunez A et al (2014) Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 73:62–68
Iqbal K, Kelly C (2015) Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther Adv Musculoskelet Dis 7:247–267
Cavagna L, Monti S, Grosso V et al (2013) The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int 2013:759760
Marino S, Sud D, Plessner H, et al. (2007) Differences in reactivation of tuberculosis induced from anti-TNF treatments are based on bioavailability in granulomatous tissue. PLoS Comput Biol [Internet] 3(10)
Alten R, Kaine J, Keystone E, Nash P, Delaet I, Genovese MC (2014) Long-term safety of subcutaneous abatacept in rheumatoid arthritis: integrated analysis of clinical trial data representing more than four years of treatment. Arthritis Rheumatol Hoboken NJ 66:1987–1997
Schiff M, Keiserman M, Codding C et al (2008) Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 67:1096–1103
Salliot C, Dougados M, Gossec L (2009) Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis 68:25–32
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ et al (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69:88–96
Buckley F, Finckh A, Huizinga TWJ, Dejonckheere F, Jansen JP (2015) Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm 21:409–423
Gabay C, Riek M, Hetland ML, et al. (2015) Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: results from a European collaborative study. Ann Rheum Dis
Favalli EG, Biggioggero M, Marchesoni A, Meroni PL (2014) Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatol Oxf Engl 53:1664–1668
Lee YH, Bae S-C (2015) Comparative efficacy and safety of tocilizumab, rituximab, abatacept and tofacitinib in patients with active rheumatoid arthritis that inadequately responds to tumor necrosis factor inhibitors: a Bayesian network meta-analysis of randomized controlled trials. Int J Rheum Dis
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Funding statement
The authors have no funding statement to declare.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Monti, S., Klersy, C., Gorla, R. et al. Factors influencing the choice of first- and second-line biologic therapy for the treatment of rheumatoid arthritis: real-life data from the Italian LORHEN Registry. Clin Rheumatol 36, 753–761 (2017). https://doi.org/10.1007/s10067-016-3528-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3528-y