Abstract
The primary objective of this study is to assess the efficacy of a Virtual Reality (VR) intervention when compared to an integrated multimodal medically managed Inpatient Program (IP) in a cohort of 24 female patients diagnosed with Bulimia Nervosa (BN). Psychological measures (i.e., EDI-2) were assessed at three points: pre-treatment, post-treatment, and at 1-month follow-up. Behavioral measures (i.e., BMI) were evaluated at 6 different time points, instead (i.e., pre-treatment, post-treatment, 3, 6, 9, and 12 months from the discharge date). The VR treatment was more effective in improving the EDI subscales EDI-DT (i.e., drive for thinness) and EDI-BU (i.e., binging-purging behaviors). In particular, patients in the VR condition showed a reduced EDI-BU score at 1-month follow-up and post-test in comparison to the pre-test, as well as a lower EDI-DT score at 1-month follow-up compared to the pre-test. Conversely, no significant changes were noted in the IP group for either subscale. Regarding the behavioral measures, the group undergoing the VR condition reported the maintenance of the BMI in the long term compared to the IP. Specifically, in the VR group BMI decreased from the pre- to post-test, and from the pre-test to the 12-month follow-up. In the IP group, BMI improved from the pre- to the post-test, and from the pre-test to the 12-month follow-up. However, a relapse pattern was observed in the IP condition during the follow-up period, with a significant BMI increase from the post-test to the 9-month follow-up, from the 3 to the 9-month follow-up, from the 6 to the 9-month follow-up, and a decrease of BMI between the 9 and the 12-month follow-up. In conclusion, these results suggest that integrating VR treatment into the care of individuals with BN could enhance both immediate and sustained treatment outcomes. This may offer valuable insights for future studies to expand and delve deeper into the field of EDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bulimia nervosa (BN) is a clinical condition belonging to the diagnostic group of Eating Disorders (EDs), characterized by frequent and repeated binge eating episodes. According to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) (American Psychiatric Association-APA, 2013), a binge eating episode is defined as the over-consumption of food during a discrete timeframe, associated with a perceived lack of control over the eating behavior, and compensatory weight control actions (i.e., self-induced vomiting, purging through the use of laxative or diuretic, severe dietary restriction and/or intense exercise). The purpose of these dysfunctional behaviors is to counteract caloric intake and prevent weight gain (Dakanalis et al. 2015).
Negative emotions often serve as triggers for binge-purging episodes (Munsch et al. 2012). Different theoretical frameworks tried to elucidate the underlying factors contributing to the manifestation of these behaviors. The affect regulation model, for example, suggests that individuals engage in binge-purging to alleviate their negative mood. In contrast, the masking theory posits that these actions serve as a way for individuals to conceal the true source of their pain by attributing it to binge-purging behaviors. Additionally, the escape theory proposes that binge-purging acts function as an “escape” mechanism to temporarily distract individuals from uncomfortable self-awareness. Although this escape may facilitate attention to immediate physical surroundings, however, it simultaneously inhibits higher-order cognitive processes, thereby reactivating suppressed binge eating behaviors (Munsch et al. 2012).
Besides binge eating episodes and purging behaviors, other two important features distinguish BN: individuals’ overvalued ideas about weight or shape (APA 2013) and a pronounced experience of body dissatisfaction (Grilo et al. 2019). Due to perceptual distortions and the bodily discomfort with their appearance, individuals with BN may indeed exhibit intense fear of gaining weight, desire to be skinny, severe body image concerns, and excessive preoccupation with their body composition, thus alternating restriction, fasting, binge eating, and purging behaviors (Zanetti 2013; Degortes et al. 2018). Consequently, bulimic patients develop attentional biases and engage in negative evaluations of their bodies, influenced by the internalization of a thinness ideal (Cash 2004). Therefore, even when presenting a normal weight, people with BN tend to overestimate their physique and perceive an increased size of the whole body or specific parts, such as the abdomen, waist, hips, and thighs (Cuzzolaro and Fassino 2018). Because of this, patients then experience low self-esteem, high drive for thinness, psychological distress, and dieting behaviors (Ruuska 2005).
In addition to attentional biases, body image distortions also correlate with a lack of interoceptive awareness (Zanetti 2013), one of the dimensions of inner body perception mostly altered in BN (Malighetti et al. 2022). According to Bruch (1962), the core symptoms of BN are indeed disruptions brought on by inaccurate perception or cognitive interpretation of bodily sensations. Disturbances in detecting and/or interpreting stimuli from the body, particularly hunger and satiety cues, could directly contribute to the development and maintenance of BN symptoms (Pollatos et al. 2012).
Cognitive Behavioral Therapy (CBT) is considered the first-line treatment for BN (Slade et al. 2018). Nevertheless, a significant portion of patients fails to respond to treatment or experiences a diminishing effect over the long term (Amianto et al. 2015; Dakanalis et al. 2017; Lampard and Sharbanee 2015; Linardon 2017).
To answer this problem, over the past decades, researchers have begun to use Virtual Reality (VR). This technology has been crucial in improving the well-being of individuals with medical conditions (e.g., Sansoni et al. 2022a, b, 2023; Sansoni and Riva 2022) and has also facilitated the integration and expansion of assessment and treatment procedures for many psychological disorders (e.g., Riva et al. 2021a, b, 2023; Brizzi et al. 2023a, b; Chorzępa et al. 2023; Vila et al. 2023), including EDs. The efficacy of VR in treating such clinical conditions stems from its capacity to involve various sensory modalities (such as visual and tactile) and manipulate spatial frames of reference (i.e., allocentric and egocentric) during task presentations. Recent research indicates that multisensory integration processes may play a pivotal role in the distorted body perceptions observed in individuals with EDs (Brizzi et al. 2023a, b), making VR an ideal solution for addressing these challenges. Common applications of VR for EDs include cue exposure, the implementation of the reference frame-shifting approach, and the utilization of body illusions (Riva et al. 2021a, b).
Integrating VR with CBT techniques has demonstrated effectiveness in enhancing motivation for change, improving self-esteem, addressing body image disturbances, and reducing binge eating and purging behaviors (Riva et al. 2021a, b). Consistent findings from randomized controlled trials (RCTs) indicate that VR-enhanced CBT (VR-CBT) surpasses CBT alone within the field of EDs (Cesa et al. 2013; Manzoni et al. 2016; Marco et al. 2013). Notably, VR-CBT not only yields faster improvements but also enhances the sustainability of therapeutic gains (Ferrer-Garcia et al. 2017; Maldonado et al. 2017; Marco et al. 2013; Perpiñá et al. 2004; Riva et al. 2003, 2004).
The goal of our study is, therefore, to test the effectiveness of VR-CBT in comparison with an inpatient multimodal treatment (IP) (i.e., TAU), to strengthen the existing knowledge on the topic, and to evaluate the use of VR to support patients with BN.
Hence, our research questions are:
RQ1: Is the VR-CBT protocol more effective than TAU in improving the attitudes and behaviors related to food, weight, and shape of patients with BN (i.e., drive for thinness, episodes of bingeing and purging, and body dissatisfaction)?
RQ2: Does the VR-CBT group, compared to TAU, provide greater BMI stability after patients’ discharge?
Following previous research (Cesa et al. 2013; Manzoni et al. 2016; Marco et al. 2013), we hypothesize that our VR-CBT protocol will be more effective than the control condition both in reducing the core psychological characteristics of BN and the BMI fluctuations over time.
2 Methods
2.1 Participants
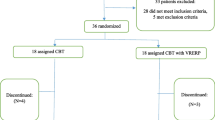
Twenty-four women diagnosed with BN according to the DSM-IV inclusion criteria were interviewed to be enrolled in this pilot study. According to Lackey and Wingate (1998), a pilot study may use a minimum of 10% of the sample required for a standard study. In a previous randomized controlled trial, Manzoni et al. (2016) performed a similar intervention with 163 patients. Taking into account this sample size, 24 subjects were considered sufficient to be included.
The study was approved by the Ethical Committee of the Istituto Auxologico Italiano and the Current Controlled Trials Organization (trial number ISRCTN59019572). Eligible criteria for participation were the following: (1) being a women aged between 18 and 50 years, (2) meeting the DSM-IV-TR criteria for BN for at least 6 months before the beginning of the research, (3) having no other severe psychiatric disorders (i.e., psychosis, depression with suicidal risk, alcohol or drug abuse), (4) not undergoing any treatment for BN, including pharmacotherapy, (5) presenting no concurrent medical condition independent to the ED, and (5) being able to provide written and informed consent to participate.
Patients meeting the inclusion criteria were randomly assigned to one of the two conditions: the integrated multimodal medically managed Inpatient Program (IP), and the VR-enhanced cognitive behavioral therapy (VR-CBT). The randomization scheme used for selecting the condition was generated by using the website www.randomization.com. The duration of both treatments was set at 5 weeks. In the IP and VR groups, CBT group sessions were always conducted by two licensed psychotherapists under the supervision of a senior professional in the field. Notably, the assignment of the two clinicians to the two treatment conditions was randomized as well. The socio-demographic and anthropometric characteristics of the two samples are summarized in Table 1.
2.2 Treatments
2.2.1 The integrated multimodal medically managed inpatient program (IP)
The integrated multimodal medically managed Inpatient Program (IP) was the standard care in the hospital where the data collection took place: for this reason, in the current study, the IP is considered the control condition. The IP consisted of 5 weeks of hospital treatment during which inpatients received medical, nutritional, physical, and psychological care. After consultation with a dietitian, all patients received a hypocaloric nutritionally balanced diet tailored to the individual (i.e., energy intake around 80% of the basal energy expenditure estimated according to the Harris-Benedict equation, and a macronutrient composition of about 16% proteins, 25% fat, and 59% carbohydrates). In addition to this, patients also followed a systematic exercise routine, attended weekly nutritional groups held by dieticians to improve and promote change in eating habits, and received psychological support in 5 weekly CBT group sessions.
2.2.2 VR-enhanced cognitive behavior therapy (VR-CBT)
Participants assigned to the VR-enhanced CBT (VR-CBT) attended 5 weekly CBT-group sessions (such as the IP condition) and 10 biweekly VR sessions of the duration of 1 h, using NeuroVR (Riva et al. 2007, 2009, 2011) (Fig. 1). Scenarios were shown in a head mounted display that participants wore during the VR-CBT. The NeuroVR software included fourteen virtual critical environments (e.g., supermarket, pub, restaurant, swimming pool, beach, gymnasium) in which patients practiced eating/relational/emotional management strategies, problem-solving, and decision-making. All triggering stimuli that produced an abnormal pattern of eating behavior (e.g., exposure to food that elicits concernst, confronting one’s body shape and weight, etc.) were assessed in the first session.
The figure presents two screenshots of the same Virtual Reality (VR) supermarket scenario, illustrating two distinct perspectives: allocentric and egocentric. Being a third-person experience, in the allocentric perspective (above) the patient’s viewpoint inludes their own body within the scenario. Conversely, in the egocentric perspective (below), the patient explores the scenario without visualizing their body, as they perceive the environment from a first-person viewpoint. Specifically, green screen videos are used in the allocentric view to tailor the VR environment to the patient’s experience
The specific VR body-image rescripting protocol developed by Riva (2011) (see Fig. 2) was used in the VR-CBT and included as part of Experiential Cognitive Therapy (ECT). During the VR sessions, the psychotherapist employed the “20/20/20 rule” (Riva 2011; Manzoni et al. 2016; Carroll 1998; Weingardt et al. 2009). During the first 20 min, the therapist focused on a clear understanding of the patient’s concerns, their level of general functioning, and food-related experiences. This part of the session tended to be characterized by patients doing most of the talk, although the clinician guided them with questions and reflections to get a sense of the patient’s current status. During the second 20 min, the emphasis was on the VR experience: the patient entered the virtual environment and faced a specific critical situation. Finally, the last 20 min of the session were spent investigating the patient’s comprehension of what occurred during the VR experience, their emotional reactions, and behavioral responses to the various circumstances encountered. New coping strategies were provided and explored as needed.
The VR body image rescripting protocol (adapted from Riva 2011)
The ten VR sessions used in our protocol had the main purpose to assess and modify the following domains:
-
Expectations and emotions related to food and weight. The clinician helped the patients identify the reasons for eating and the functional coping strategies concerning their specific emotional/behavioral triggers. Several CBT methods (i.e., countering, alternative interpretation, label shifting, deactivating the illness belief) could be used to accomplish this goal.
-
Strategies employed to cope with difficult interpersonal and potential maintenance situations. Through the use of temptation exposure with response prevention, and by working on three key dimensions (i.e., perceived control, perceived competence, and goal internalization) patients practiced new skills and behaviors.
-
Body experience of the subject. A specific VR body mage rescripting protocol was used to update the patient’s memory of the body (Riva 2011; Riva et al. 2001). In the protocol (Fig. 2) different body-related situations were experienced both from a first-person (i.e., egocentric) and third-person (i.e., allocentric) perspective, integrating the therapeutic methods used by Butters and Cash (1987) and Wooley and Wooley (1985). The clinician solicited the patient to provide detailed descriptions of the virtual experience and the feelings associated with it. Furthermore, the patient was taught how to cope with them using different techniques.
In all the sessions, the psychotherapist adopted a Socratic style: the use of questions pertaining the contents of the virtual world allowed indeed patients to synthesize information and draw conclusions on their own.
2.3 Measures
Patients considered eligible for the study were invited for a first interview during which informed written consent was completed. Height was measured with a stadiometer, and weight was assessed on a balance beam scale, with the participant in lightweight clothing and with shoes removed. Assessments were obtained at pre-treatment (i.e., before VR-CBT or IP), post-treatment (during the last week of hospitalization, after the intervention completion), and at a 1-month follow-up for the psychological measures, and at six follow-up time-points (i.e., pre-treatment, post-treatment, at 3, 6, 9 and 12-months post-treatment) for the behavioral measures.
Psychological measures included the Eating Disorder Inventory-2 (EDI-2) (Garner 1991—Italian validation by Rizzardi, Trombini & Trombini 1995): the EDI-2 is a self-report questionnaire that captures the essence and heterogeneity of EDs by assessing the attitudes and behaviors related to food, weight, and shape (subscales Drive for Thinness (DT), Bulimia (BU), and Body Dissatisfaction (BD)), and investigating general psychological traits related to EDs (subscales Ineffectiveness (IN), Perfectionism (PE), Interpersonal Distrust (ID), Interoceptive Awareness (IA) and Maturity Fears (MF)), as well as additional general features (subscales Asceticism (AS), Impulse Regulation (IR), and Social Insecurity (SI)). The 91 items that compose the EDI-2 are rated on a six-point Likert-type scale, using a 3-point system where “sometimes”, “rarely” and “never” are assigned zeros while “often”, “usually”, and “always” are assigned a score of 1, 2 and 3, respectively. As has been done in other research (Stein et al. 2015), and given the purpose of the current study, we decided to administer only three EDI-2 subscales: DT, BU, and BD. The DT subscale measures concern with dieting, preoccupation with weight, and fear of weight gain; the BU subscale evaluates episodes of bingeing and purging; the BD subscale assesses dissatisfaction with one’s physical appearance. Higher scores are indicative of dysfunctional behavior.
Behavioral measures included the assessment of the Body Mass Index (BMI), instead.
2.4 Statistical analyses
The software R version 3.6.3 (R Core Team 2014) was used to perform the statistical analyses of this study. In particular, to assess the effect of the two treatments (IP vs VR-CBT) on both psychological (i.e., EDI-2) and behavioral measures (i.e., BMI) over the different time points, we used the lmer function from the lme4 package in R (version 3.6.3), with the optimizer set to “bobyqa” and the REML (Restricted Maximum Likelihood) estimation method. Most of the dependent variables at the pre-test were normally distributed in the two groups, thus we proceeded with parametric methods. A Type III Analysis of Covariance (ANCOVA) table with Satterthwaite’s method was employed to assess the significance of each fixed effect in the model. All models had a random intercept for the individual ID to account for within-subject variability. Post-hoc analyses were performed using the estimated marginal means method with Bonferroni correction. Partial eta squared (η2p) was interpreted and used as a metric of effect size (small = 0.01, medium = 0.06, and large = 0.14), and the significance level (Alpha level) for all the analyses was set to 0.05. For the psychological measures (i.e., EDI-2) we used a 2 (experimental and control group) × 3 (three time-points—pre-treatment, post-treatment, and at 1-month follow-up) design, while for the behavioral measures (i.e., BMI) we employed a 2 (experimental and control group) × 6 (six time-points—pre-treatment, post-treatment, 3, 6, 9 and 12-months post-treatment) design.
3 Results
3.1 Psychological measures
To evaluate the effectiveness of VR-CBT we looked at the difference between the control (i.e., IP) and the experimental group (i.e., VR-CBT) in BN-related measures at pre-treatment, post-treatment, and 1-month follow-up while controlling for baseline BMI. The linear mixed-effects ANCOVAs revealed a significant interaction for most of the variables of interest.
Specifically, concerning the subscale EDI-BU, we found a main effect of time (F2,44 = 3.27, p = 0.047, η2p = 0.13) and an interaction effect between time and group (F2,44 = 6.29, p = 0.004, η2p = 0.22). Regarding the VR condition, post-hoc analyses showed lower scores at 1-month follow-up (adjusted mean difference = − 6.5, SE = 1.7, p = 0.001) and post-test (adjusted mean difference = − 5.58, SE = 1.7, p = 0.006) compared to the pre-test. No significant results were found in the control group, instead.
About the subscale EDI-DT, we only found an interaction effect between time and group (F2,44 = 5.67, p = 0.006, η2p = 0.2). Post-hoc analyses displayed lower scores at 1-month follow-up (adjusted mean difference = − 4.33, SE = 1.23, p = 0.003) compared to the pre-test in the VR group. No significant results were found in the IP group.
Lastly, when considering the subscale EDI-BD we found a main effect of the covariate BMI at baseline (F1,21 = 6.5, p = 0.019, η2p = 0.24) and an interaction effect between time and group (F2,44 = 3.87, p = 0.028, η2p = 0.15). However, post-hoc analyses showed no significant results in the VR condition and control group (Fig. 3).
3.2 Behavioral measures
A linear mixed-effects ANCOVA was conducted to examine the effects of time (pre, post, follow-up at 3, 6, 9, and 12 months), group (VR Vs. IP), and their interaction on BMI while controlling for BMI at baseline (Fig. 4).
We found a significant effect of time on BMI (F5, 110 = 6.68, p < 0.001, h2p = 0.23), while the main effect of the group approached significance (F1, 21 = 3.44, p = 0.078, h2p = 0.14). The effect of BMI at baseline was highly significant (F1, 21 = 642.9, p < 0.001), suggesting that BMI at baseline consistently influenced the outcome measures. Furthermore, the interaction between time and group was also significant (F5, 110 = 3.55, p = 0.005, h2p = 0.14), indicating that the effect of time on BMI varied depending on the group. Post-hoc analyses adjusted with Bonferroni correction showed that BMI in the VR group decreased from pre to post-test (adjusted estimated difference = − 1.59, SE = 0.52, p = 0.046) and from pre-test to the 12-month follow-up (adjusted estimated difference = − 1.59, SE = 0.52, p = 0.046). In the IP group, we found that the control intervention reduced the BMI from pre to post-test (adjusted estimated difference = − 1.58, SE = 0.52, p = 0.047) and from pre-test to the 12-month follow-up (adjusted estimated difference = − 1.58, SE = 0.52, p = 0.047). However, we also found a relapse pattern during the follow-up period. BMI significantly increased from post-test to 9-month follow-up (adjusted mean difference = 2.49, SE = 0.52, p < 0.001), from 3-month to 9-month follow-up (adjusted mean difference = − 2.12, SE = 0.52, p = 0.001), from 6-month to 9-month follow-up (adjusted mean difference = − 2.04, SE = 0.52, p = 0.003), and decrease between 9-month and 12-month follow-up (adjusted estimated difference = − 2.49, SE = 0.52, p < 0.001). Nevertheless, the average delta in the VR and control groups between the pre-test and 12-month follow-up was -1.6 (Fig. 5).
4 Discussion
This pilot study aimed to test the effectiveness of a VR-CBT intervention in managing core symptoms of BN by comparing the experimental condition (VR-CBT) to the TAU (i.e., IP). In line with previous research (e.g., Cesa et al. 2013; Manzoni et al. 2016; Marco et al. 2013; Ferrer-García 2019), our results showed the effectiveness of the VR-CBT protocol in decreasing the preoccupation with weight and fear of weight gain, as well as reducing the episodes of binge eating and purging. In addition to this, participants in the VR-CBT condition reported a more stable BMI over time compared to the control arm, in which patients had significantly more BMI fluctuations.
Specifically, within the IP group, the BMI showed a significant increase after the end of the treatment (between several follow-up points), followed by a consistent decrease between 9 and 12 months after discharge. Although most BN patients fall in the normal weight range when they first seek therapy, many may have reported a higher BMI before. Individuals, in fact, often experience a substantial weight loss while their disease develops and their need for support becomes inevitable. Because of this, patients may perceive weight increases as a risk of regaining their prior peak weight, thus rendering them vulnerable to reacting to weight fluctuations with an increase in maladaptive compensatory behaviors (Juarascio et al. 2018). The pattern that we observed in the control condition (i.e., weight increase followed by a significant weight loss) could support the idea that the weight reduction reported could be the manifestation of compensatory behaviors that may have not taken place in the VR-CBT group, in which we witnessed a lessening of binge-purging behaviors and a lower drive for thinness, indeed. Therefore, the potential of our VR protocol to stabilize weight fluctuations over time seems particularly promising in the treatment of BN.
A possible explanation for the greater effectiveness of the VR protocol than TAU in regulating most symptoms of BN might be related to the Allocentric Lock Theory (ALT) (Riva 2014; Riva and Dakanalis 2018; Riva and Gaudio 2018). According to this hypothesis, EDs are a consequence of impairments in the way the body is perceived and remembered (Gaudio and Riva 2013; Riva 2011; Riva and Gaudio 2012; Riva et al. 2015). In this sense, EDs may be the result of a multisensory processing deficit in the way expected versus experienced body-related experiences are integrated (Brizzi et al. 2023a, b). Specifically, this theory posits two possible alterations: (1) in the ability to link interoceptive bodily signals to their potential consequences; and (2) to update the mnestic representation of the body (i.e., allocentric memory) with new contents driven from perception (i.e., egocentric perceptual-driven inputs) (Riva and Dakanalis 2018; Riva and Gaudio 2018). This dual impairment may lead individuals with EDs to be locked within a negative representation of their body that cannot be updated with real-time bodily perceptions (Manzoni et al. 2016). The consequences of this cognitive bias consist of the permanent experience of a wrong and overestimated body, disregarding the evidence of the actual shape or size; this compels individuals with EDs to undergo an experience of a body that diverges from their real one. From a cognitive perspective, the allocentric memory changes and reorganizes the existing memories of the body producing a priming effect on any bodily experience that affects thoughts and behaviors involved in the interpretation of any future body-relevant events (Cipolletta et al. 2017; Malighetti et al. 2016; Riva et al. 2001; Serino et al. 2015). In our study, the VR intervention utilizes an embodied technology (Spagnolli and Gamberini 2005) capable of correcting inaccurate body memories. By immersing participants in both first-person and third-person perspectives, the VR protocol effectively releases the negative representation and modifies its behavioral and emotional counterparts. Essentially, it functions as a sensory training to unlock the body’s memory through a body image rescripting process, enhancing the input of new egocentric/internal somatosensory information directly associated with the existing allocentric memory (Cesa et al. 2013; Manzoni et al. 2016).
Despite its results, however, our study has some limitations. First of all, the small sample size. Our VR-CBT group reported an improvement in all the psychological variables of interest but body dissatisfaction. Indeed, even if the results revealed a significant interaction between time and group, this finding did not persist after post-hoc analyses. A plausible explanation could be that this sample size probably did not provide enough power for the interaction to last. In addition to this, presenting a sample size of 24 complicates the generalization of our study results to the entire population of individuals with BN. For both these reasons, further research is necessary to replicate this work with a larger sample. This would allow to examine not only if the same outcomes still stand, but also if our VR protocol might have a favorable impact on body dissatisfaction, too. Secondly, to make the administration more agile, we have chosen to administer only the specific subscales of the EDI-2 strictly related to the study objectives. Employing a more extensive set of psychological features may contribute to a deeper comprehension of the treatment’s effects. For the same reason, during follow-ups, we only asked for objective data on BN-related behavioral measures (i.e., BMI): data on psychological variables are missing. Additional studies are thus indispensable to evaluate both if the VR intervention has an impact on other psychological areas (e.g., depression and/or anxiety) (e.g., Fernández-Álvarez et al. 2020, 2021), and if the benefits of VR on such measures are maintained in the long term (e.g., 12 months after discharge). Lastly, according to the study by Malighetti et al. (2022), patients with EDs frequently exhibit deficits in interoception, proprioception, and vestibular signals. This finding raises the possibility that VR needs to simulate and modify both the external and internal body to be effective with these patients, especially in the long term (Riva et al. 2021c). In our study, only the external body is simulated and corrected by the VR intervention; the internal body (e.g., inner body perceptions), which also appears to be a key factor in the etiology of EDs (e.g., Di Lernia et al. 2019), is not. The way we perceive the body is the consequence of integrating a variety of biological signals that have to be controlled and matched a) from the outside (exteroception, the body as experienced through the senses), (b) from the inside (interoception—the sense of the physiological conditions of the body, proprioception—the sense of the position of the body/body segments, and vestibular input—the sense of movement of the body), and from the memory (Riva 2018). The new approach called Regenerative Virtual Medicine (RVM), combines VR with other somatic modification approaches (i.e., interoceptive, and brain stimulation technologies) capable of addressing and modifying our inner body experience (Riva et al. 2021a, b). This may offer a promising solution to this issue. For this reason, future trials should consider the possibility of including RVM in their protocols to advance the treatment of EDs and target in an all-encompassing way the multidimensional nature of EDs (Malighetti et al. 2022).
5 Conclusions and main contributions of the study
Despite the study limitations discussed above, our findings support the hypothesis that the integration of a VR-based intervention, aimed at both unlocking the negative memory of the body and modifying its behavioral and emotional correlates, could enhance both short and long-term outcomes in the treatment of BN.
Over and above the introduction of an additional treatment strategy for BN, the present study makes noteworthy contributions to the field of EDs, with particular emphasis on two key areas: replicability and specificity.
The former refers to the replication crisis that psychology is suffering, and to the possibility through this work to replicate previous results (e.g., Cesa et al. 2013). Extant literature has stressed the importance of replicating outcomes of former studies to advance knowledge, and improve research practice (e.g., Maxwell et al. 2015; Shrout and Rodgers 2018; Tackett et al. 2019). Even though other researchers have used VR paradigms employing NeuroVR (e.g., Cesa et al. 2013; Gorini et al. 2010) or different VR-CBT protocols (Riva et al. 2021a, b), it is necessary to boost confidence that the treatment outcomes related to these protocols genuinely exist and are not false positives (Diener and Biswas-Diener 2024). This process holds particular significance when discussing interventions targeted at enhancing clinical conditions. It is indeed crucial for clinicians to ascertain the reliability and effectiveness of treatments, for policymakers to determine if specific TAU approaches should be replaced with more efficacious interventions, and for scientists to identify areas requiring further in-depth analysis to enhance existing therapies and push the boundaries of the field.
The second contribution of our pilot is the specificity of our sample. As shown by Clus et al. (2018), studies using VR exclusively in patients with BN are at an early stage. Researchers, in fact, usually test interventions on patients with different diagnoses, mostly including within the same sample individuals with BN and Anorexia Nervosa (AN) (e.g., Marco et al. 2013; Serino et al. 2015), or people with BN and Binge Eating Disorder (BED) (e.g., De Carvalho et al. 2017; Ferrer-Garcia et al. 2019). However, BN, AN, and BED are extremely different disorders, whose specificity needs to be considered for appropriate therapeutic implications. If we consider, for example, the two main psychological outcomes of our study (i.e., reduction of binge-purging episodes and drive for thinness) we can easily see how BN, AN, and BED differ. Antecedents of binge eating in BED seem to be dissimilar to the binge cycle of BN in terms of emotion regulation and mechanisms that drive binge eating episodes, even if both conditions share the binging behavior as one of the core symptoms characterizing the disorder (Munsch et al. 2012). Regarding the drive for thinness, the study by Barry et al. (2003) displays how this feature is consistently distinct among patients with BN and obese patients with BED, with the latter group of patients reporting a significantly lower drive for thinness than the former one. As reported by the authors, these results support earlier research demonstrating that patients with BN have higher levels of food constraint than patients with BED and that the degree of dietary restraint in patients with BED is linked to obesity (Barry et al. 2003). Drive for thinness is also different when considering anorectic patients. One of the main distinctive elements of AN, shared with many bulimic patients, is the fear of gaining weight, which consequently causes a drive for and pursuit of thinness. Surprisingly, however, research highlights the presence of atypical anorectic patients who do not present such features (Abbate-Daga et al. 2007). Even if it is paradoxical that patients with AN report a low score of drive for thinness, in the study by Abbate-Daga et al. (2007) this group was quite consistent and represented 38% of the whole sample, revealing that the absence of drive for thinness in AN is a recurring phenomenon. Due to the high diversification of EDs, treating individuals with different disorders as an aggregate group can thus be confusing and lead to inaccurate results.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abbate-Daga G, Pierò A, Gramaglia C, Gandione M, Fassino S (2007) An attempt to understand the paradox of anorexia nervosa without drive for thinness. Psychiatry Res 149(1–3):215–221. https://doi.org/10.1016/j.psychres.2005.10.017
Amianto F, Ottone L, Abbate Daga G, Fassino S (2015) Binge-eating disorder diagnosis and treatment: a recap in front of DSM-5. BMC Psychiatry 15:70. https://doi.org/10.1186/s12888-015-0445-6
American Psychological Association-APA (2013) Diagnostic and statistical manual of mental disorders (5th. Ed.). APA, Washington, DC
Barry DT, Grilo CM, Masheb RM (2003) Comparison of patients with bulimia nervosa, obese patients with binge eating disorder, and nonobese patients with binge eating disorder. J Nerv Ment Dis 191(9):589–594. https://doi.org/10.1097/01.nmd.0000087185.95446.65
Brizzi G, Sansoni M, Riva G (2023a) The BODY-FRIEND project: using new technology to learn about how people with anorexia feel about their bodies. Cyberpsychol Behav Soc Netw. https://doi.org/10.1089/cyber.2023.29267.ceu
Brizzi G, Sansoni M, Di Lernia D et al (2023b) The multisensory mind: a systematic review of multisensory integration processing in Anorexia and Bulimia nervosa. J Eat Disord 11:204. https://doi.org/10.1186/s40337-023-00930-9
Bruch H (1962) Perceptual and conceptual disturbances in Anorexia nervosa. Psychosom Med 24(2):187–194
Butters JW, Cash TF (1987) Cognitive-behavioral treatment of women’s body image satisfaction: a controlled outcomestudy. J Consult Clin Psychol 55:889–897
Carroll K (1998) A cognitive-behavioral approach: treating cocaine addiction, vol 1. US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse
Cash TF, Pruzinsky T (2004) Body image: a handbook of theory, research, and clinical practice. The Guilford Press
Cesa GL et al (2013) Virtual reality for enhancing the cognitive behavioral treatment of obesity with binge eating disorder: randomized controlled study with one-year follow-up. J Med Internet Res 15:e113. https://doi.org/10.2196/jmir.2441
Chorzępa M, Sansoni M, De Gaspari S, Riva G (2023) Tricking your brain into loving your body: the integrated power of body illusions and interoceptive technologies. Cyberpsychol Behav Soc Netw. https://doi.org/10.1089/cyber.2023.29281.ceu
Cipolletta S, Malighetti C, Serino S, Riva G, Winter D (2017) Intrapersonal, interpersonal, and physical space in anorexia nervosa: a virtual reality and repertory grid investigation. Psychiatry Res 252:87–93. https://doi.org/10.1016/j.psychres.2017.02.060
Clus D, Larsen ME, Lemey C, Berrouiguet S (2018) The use of virtual reality in patients with eating disorders: systematic review. J Med Internet Res 20(4):e157. https://doi.org/10.2196/jmir.7898
Cuzzolaro M, Fassino S (2018) Body image, eating, and weight. A guide to assessment, treatment, and prevention. Springer, Cham
Dakanalis A, Carra G, Calogero R, Fida R, Clerici M, Zanetti MA, Riva G (2015) The developmental effects of media-ideal internalization and self-objectification processes on adolescents’ negative body-feelings, dietary restraint, and binge eating. Eur Child Adolesc Psychiatry 24:997–1010. https://doi.org/10.1007/s00787-014-0649-1
Dakanalis A, Colmegna F, Riva G, Clerici M (2017) Validity and utility of the DSM-5 severity specifier for binge-eating disorder. Int J Eat Disord 50:917–923. https://doi.org/10.1002/eat.22696
De Carvalho MR, Dias TRDS, Duchesne M, Nardi AE, Appolinario JC (2017) Virtual reality as a promising strategy in the assessment and treatment of bulimia nervosa and binge eating disorder: a systematic review. Behav Sci 7(3):43. https://doi.org/10.3390/bs7030043
Degortes D, Santonastaso P, Favaro A (2018) Body image disturbances in Bulimia nervosa. Body image, eating, and weight. Springer, Cham
Diener E, Biswas-Diener R (2024) The replication crisis in psychology. In: Biswas-Diener R, Diener E (eds) Noba textbook series: Psychology. DEF publishers, Champaign, IL. http://noba.to/q4cvydeh
Di Lernia D, Serino S, Polli N, Persani L, Riva G (2019) Interoceptive axes dissociation in anorexia nervosa: a single case study with follow up post-recovery assessment. Front Psychol 9:401591. https://doi.org/10.3389/fpsyg.2018.02488
Fernández-Álvarez J, Di Lernia D, Riva G (2020) Virtual reality for anxiety disorders: rethinking a field in expansion. In: Kim YK (ed) Anxiety disorders. Advances in experimental medicine and biology, vol 1191. Springer, Singapore. https://doi.org/10.1007/978-981-32-9705-0_21
Fernandez-Alvarez J, Colombo D, Suso-Ribera C, Chirico A, Serino S, Di Lernia D et al (2021) Using virtual reality to target positive autobiographical memory in individuals with moderate-to-moderately severe depressive symptoms: a single case experimental design. Internet Interv 25:100407. https://doi.org/10.1016/j.invent.2021.100407
Ferrer-Garcia M et al (2017) A randomised controlled comparison of second-level treatment approaches for treatmentresistant adults with bulimia nervosa and binge eating disorder: assessing the benefits of virtual reality cue exposure therapy. Eur Eat Disord Rev. https://doi.org/10.1002/erv.2538
Ferrer-García M, Pla-Sanjuanelo J, Dakanalis A, Vilalta-Abella F, Riva G, FernandezAranda F, Forcano L, Riesco N, Sánchez I, Clerici M, Ribas-Sabaté J (2019) A randomized trial of virtual reality-based cue exposure second-level therapy and cognitive behavior second-level therapy for bulimia nervosa and binge-eating disorder: Outcome at six-month followup. Cyberpsychol Behav Soc Netw 22:60–68. https://doi.org/10.1089/cyber.2017.0675
Garner DM (1991) Eating Disorder Inventory-2. Professional manual. Psychological Assessment Research Inc., Odessa, Florida
Gaudio S, Riva G (2013) Body image in anorexia nervosa: the link between functional connectivity alterations and spatial reference frames. Biol Psychiatry 73:e25-26. https://doi.org/10.1016/j.biopsych.2012.08.028
Gorini A, Griez E, Petrova A, Riva G (2010) Assessment of the emotional responses produced by exposure to real food, virtual food and photographs of food in patients affected by eating disorders. Ann Gen Psychiatry 9(1):1–10. https://doi.org/10.1186/1744-859X-9-30
Grilo CM, Crosby RD, Machado PP (2019) Examining the distinctiveness of body image concerns in patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord 52(11):1229–1236. https://doi.org/10.1002/eat.23161
Juarascio A, Lantz EL, Muratore AF, Lowe MR (2018) Addressing weight suppression to improve treatment outcome for Bulimia nervosa. Cogn Behav Pract 25(3):391–401
Lackey NR, Wingate AL (1998) The pilot study: One key to research success. In: Brink PJ, Wood MJ (eds) Advanced design in nursing research, 2nd edn. Sage, Thousand Oaks, CA
Lampard AM, Sharbanee JM (2015) The Cognitive-behavioural theory and treatment of Bulimia nervosa: an examination of treatment mechanisms and future directions. Aust Psychol 50:6–13. https://doi.org/10.1111/ap.12078
Linardon J, Wade TD, de la Piedad Garcia X, Brennan L (2017) The efficacy of cognitive-behavioral therapy for eating disorders: a systematic review and meta-analysis. J Consult Clin Psychol 85(11):1080
Maldonado JG, Ferrer-Garcia M, Dakanalis A, Riva G (2017) Virtual reality: applications to eating disorders. In: Agras SW, Robinson AH (eds) The Oxford handbook of eating disorders, 2nd edn. Oxford University Press
Malighetti C, Sansoni M, Gaudio S, Matamala-Gomez M, Di Lernia D, Serino S, Riva G (2022) From virtual reality to regenerative virtual therapy: some insights from a systematic review exploring inner body perception in Anorexia and Bulimia nervosa. J Clin Med 11:7134. https://doi.org/10.3390/jcm11237134
Malighetti C, Serino S, Riva G, Cipolletta S (2016) Inside and outside the self. Virtual reality and repertory grids in the spatial analysis of anorexic patients’ meanings. Annu Rev CyberTherapy Telemed 14:78–81
Manzoni GM et al (2016) Virtual reality–enhanced cognitive–behavioral therapy for morbid obesity: a randomized controlled study with 1 year follow-up. Cyberpsychol Behav Soc Netw 19:134–140. https://doi.org/10.1089/cyber.2015.0208
Marco JH, Perpina C, Botella C (2013) Effectiveness of cognitive behavioral therapy supported by virtual reality in the treatment of body image in eating disorders: one year follow-up. Psychiatry Res 209:619–625. https://doi.org/10.1016/j.psychres.2013.02.023
Maxwell SE, Lau MY, Howard GS (2015) Is psychology suffering from a replication crisis? What does “failure to replicate” really mean? Am Psychol 70(6):487
Munsch S, Meyer AH, Quartier V, Wilhelm FH (2012) Binge eating in binge eating disorder: a breakdown of emotion regulatory process? Psychiatry Res 195(3):118–124. https://doi.org/10.1016/j.psychres.2011.07.016
Perpiñá C, Marco JH, Botella C, Banos R (2004) Tratamiento de laimagen corporal enlostrastornosalimentarios mediante tratamiento cognitivo-comportamental apoyadoconrealidad virtual: Resultados al ano de seguimiento. Psicol Conductual 12:519–537
Pollatos O, Fustos J, Critchley HD (2012) On the generalised embodiment of pain: how interoceptive sensitivity modulates cutaneous pain perception. Pain 153:1680–1686. https://doi.org/10.1016/j.pain.2012.04.030
Riva G (2011) The key to unlocking the virtual body: virtual reality in the treatment of obesity and eating disorders. J Diabetes Sci Technol. https://doi.org/10.1177/193229681100500213
Riva G (2014) Out of my real body: cognitive neuroscience meets eating disorders. Front Hum Neurosci 8:236. https://doi.org/10.3389/fnhum.2014.00236
Riva G (2018) The neuroscience of body memory: From the self through the space to the others. Cortex 104:241–260
Riva G, Dakanalis A (2018) Altered processing and integration of multisensory bodily representations and signals in eating disorders: a possible path toward the understanding of their underlying causes. Front Hum Neurosci 12:49. https://doi.org/10.3389/fnhum.2018.00049
Riva G, Gaudio S (2012) Allocentric lock in anorexia nervosa: new evidences from neuroimaging studies. Med Hypotheses 79:113–117. https://doi.org/10.1016/j.mehy.2012.03.036
Riva G, Gaudio S (2018) Locked to a wrong body: eating disorders as the outcome of a primary disturbance in multisensory body integration. Conscious Cogn 59:57–59. https://doi.org/10.1016/j.concog.2017.08.006
Riva G, Bacchetta M, Baruffi M, Molinari E (2001) Virtual reality–based multidimensional therapy for the treatment of body image disturbances in obesity: a controlled study. Cyberpsychol Behav 4(4):511–526
Riva G, Bacchetta M, Cesa G, Conti S, Molinari E (2003) Six-month follow-up of in-patient experiential cognitive therapy for binge eating disorders. Cyberpsychol Behav 6:251–258. https://doi.org/10.1089/109493103322011533
Riva G, Bacchetta M, Cesa G, Conti S, Molinari E (2004) The use of VR in the treatment of eating disorders. Stud Health Technol Inf 99:121–163
Riva G, Gaggioli A, Villani D, Preziosa A, Morganti F, Corsi R et al (2007) NeuroVR: an open source virtual reality platform for clinical psychology and behavioral neurosciences. In: MMVR, pp 394–399
Riva G, Carelli L, Gaggioli A, Gorini A, Vigna C, Algeri D et al (2009) NeuroVR 1.5 in practice: actual clinical applications of the open source VR system. Annu Rev Cybertherapy Telemed 144:57–60
Riva G, Gaggioli A, Grassi A, Raspelli S, Cipresso P, Pallavicini F, Vigna C, Gagliati A, Gasco S, Donvito G (2011) NeuroVR 2–a free virtual reality platform for the assessment and treatment in behavioral health care. Medicine meets virtual reality 18. IOS Press, pp 493–495
Riva G, Gaudio S, Dakanalis A (2015) The neuropsychology of self-objectification. Eur Psychol 20:34–43. https://doi.org/10.1027/1016-9040/a000190
Riva G, Serino S, Di Lernia D, Pagnini F (2021a) Regenerative virtual therapy: the use of multisensory technologies and mindful attention for updating the altered representations of the bodily self. Front Syst Neurosci 15:749268
Riva G, Malighetti C, Serino S (2021b) Virtual reality in the treatment of eating disorders. Clin Psychol Psychother 28(3):477–488. https://doi.org/10.1002/cpp.2622
Riva G, Di Lernia D, Sajno E, Sansoni M, Bartolotta S, Serino S et al (2021c) Virtual reality therapy in the metaverse: merging VR for the outside with VR for the inside. Annu Rev Cybertherapy Telemed 19:3–8
Riva G, Di Lernia D, Tuena C, Bernardelli L, Maldonado JG, Ferrer-Garcia M, Porras-Garcia B, Meyer ML, Shiban Y, Castelnuovo G, Pagnini F, Pedroli E, Sforza F, Clementi A, Sansoni M, Wiederhold BK, Serino S (2023) A self-help virtual therapeutic experience intervention for overcoming psychological distress related to the COVID-19 pandemic: results from the European multicentric COVID feel good trial. Psychosomat Med 85(7):639–650. https://doi.org/10.1097/PSY.0000000000001198
Rizzardi M, Trombini E, Trombini G (1995) EDI-2, Eating Disorder Inventory-2. Adattamento italiano, O.S., Organizzazioni Speciali, Firenze
Ruuska J, Kaltiala-Heino R, Rantanen P, Koivisto AM (2005) Are there differences in the attitudinal body image between adolescent anorexia nervosa and bulimia nervosa? Eat Weight Disord-Stud Anorexia Bulimia Obes 10(98):106
Sansoni M, Riva G (2022) 360-VIRTOncology: virtual reality to improve cancer patients’ well-being. Cyberpsychol Behav Soc Netw 25(9):620–622. https://doi.org/10.1089/cyber.2022.29255.ceu. (PMID: 36108284)
Sansoni M, Scarzello G, Serino S, Groff E, Riva G (2022a) Mitigating negative emotions through virtual reality and embodiment. Front Hum Neurosci 16:916227. https://doi.org/10.3389/fnhum.2022.916227
Sansoni M, Malighetti C, Riva G (2022b) Psychological and educational interventions among cancer patients: a systematic review to analyze the role of immersive virtual reality for improving patients’ well-being. In: De Paolis LT, Arpaia P, Sacco M (eds) Extended reality. XR Salento 2022. Lecture notes in computer science. Springer, Cham. https://doi.org/10.1007/978-3-031-15553-6_30
Sansoni M, Brizzi G, Vila A, Guillen-Sanz H, Strocchia F, De Gaspari S, Sajno E, Riva G (2023) Looking through your eyes: using immersive virtual reality to promote well-being among cancer survivors and their partners. Annu Rev Cyberther Telemed 21:185–190
Schwartz MB, Brownell KD (2004) Obesity and Body image. Body Image 1:43–56
Serino S, Dakanalis A, Gaudio S, Carra G, Cipresso P, Clerici M, Riva G (2015) Out of body, out of space: impaired reference frame processing in eating disorders. Psychiatry Res 230:732–734. https://doi.org/10.1016/j.psychres.2015.10.025
Shrout PE, Rodgers JL (2018) Psychology, science, and knowledge construction: broadening perspectives from the replication crisis. Annu Rev Psychol 69(1):487–510
Slade PD, Dewey ME, Newton T, Brodie D, Kiemle G (1990) Development and preliminary validation of the body satisfaction scale (BSS). Psychol Health 4:213–220
Slade E, Keeney E, Mavranezouli I, Dias S, Fou L, Stockton S, Saxon L, Waller G, Turner H, Serpell L, Kendall T (2018) Treatments for bulimia nervosa: a network meta-analysis. Psychol Med 48(16):2629–2636. https://doi.org/10.1017/S0033291718001071
Spagnolli A, Gamberini L (2005) A Place for presence. Understanding the human involvement in mediated interactive environments. Psychol J 3(1):6–15
Stein KF, Riley BB, Hoyland-Domenico L, Lee CK (2015) Measurement of body dissatisfaction in college-enrolled Mexican American women: a rasch-based examination of the validity and reliability of the EDI-III. Eat Behav 19:5–8. https://doi.org/10.1016/j.eatbeh.2015.06.001
Stice E, Agras WS (1998) Predicting onset and cessation of bulimic behaviors during adolescence: a longitudinal grouping analysis. Behav Ther 29(2):257–276
Tackett JL, Brandes CM, King KM, Markon KE (2019) Psychology’s replication crisis and clinical psychological science. Annu Rev Clin Psychol 15:579–604
Vila A, Ardoy-Cuadros J, Romero-Moreno R, Nogales-Gonzalez C (2023) Novel therapeutic and educational approaches: using technology to improve sexuality. In: Sterling R (ed) Sexual education around the world - past, present and future issues. Springer, Cham, pp 103–117. https://doi.org/10.5772/intechopen.1001854
Weingardt KR, Cucciare MA, Bellotti C, Lai WP (2009) A randomized trial comparing two models of web-based training in cognitive–behavioral therapy for substance abuse counselors. J Subst Abuse Treat 37(3):219–227
Wooley SC, Wooley OW (1985) Intensive out-patient and residential treatment for Bulimia. In: Garner DM, Garfinkel PE (eds) Handbook of psychotherapy for Anorexia and Bulimia. Guil- ford Press, New York, pp 120–132
Zanetti T, Santonastaso P, Sgaravatti E, Degortes D, Favaro A (2013) Clinical and temperamental correlates of body image disturbance in eating disorders. Eur Eat Disord Rev 21:32–37
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This research was funded by (a) the Italian Ministry of Health—Ricerca Corrente; (b) the research project “Arcadia VR—Assistenza e Riabilitazione del Compor-tamento Alimentare tramite Dispositivi basati sull’Intelligenza Artificiale e Realtà Virtuale” (Arcadia VR —Assistance and Rehabilitation of Eating Behaviour through Artificial Intelligence-based Devices and Virtual Reality)—MISE (Prog. N.ro F/310025/01–05/X56), Fondo crescita sostenibile—Accordi per l’Innovazione, ai sensi del Decreto Ministeriale del 31 dicembre 2021 e del successivo DD 721 del 18 marzo 2022, N. Pos. 25; (c) The Italian MIUR research project “Unlocking the memory of the body: Virtual Reality in Anorexia, 201597WTTM. Lastly, open access funding is provided by the Catholic University of Sacred Heart of Milan, within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sansoni, M., Varallo, G., Malighetti, C. et al. Unlocking the potential of virtual reality to expand treatment frontiers for bulimia nervosa: a pilot study to explore the impact of virtual reality-enhanced cognitive-behavioral therapy. Virtual Reality 28, 79 (2024). https://doi.org/10.1007/s10055-024-00971-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10055-024-00971-8