Abstract
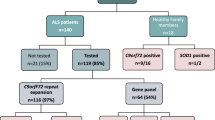
Hereditary spastic paraplegia (HSP) is a group of neurodegenerative diseases with a high genetic and clinical heterogeneity. Numerous HSP patients remain genetically undiagnosed despite screening for known genetic causes of HSP. Therefore, identification of novel variants and genes is needed. Our previous study analyzed 74 adult Serbian HSP patients from 65 families using panel of the 13 most common HSP genes in combination with a copy number variation analysis. Conclusive genetic findings were established in 23 patients from 19 families (29%). In the present study, nine patients from nine families previously negative on the HSP gene panel were selected for the whole exome sequencing (WES). Further, 44 newly diagnosed adult HSP patients from 44 families were sent to WES directly, since many studies showed WES may be used as the first step in HSP diagnosis. WES analysis of cohort 1 revealed a likely genetic cause in five (56%) of nine HSP families, including variants in the ETHE1, ZFYVE26, RNF170, CAPN1, and WASHC5 genes. In cohort 2, possible causative variants were found in seven (16%) of 44 patients (later updated to 27% when other diagnosis were excluded), comprising six different genes: SPAST, SPG11, WASCH5, KIF1A, KIF5A, and ABCD1. These results expand the genetic spectrum of HSP patients in Serbia and the region with implications for molecular genetic diagnosis and future causative therapies. Wide HSP panel can be the first step in diagnosis, alongside with the copy number variation (CNV) analysis, while WES should be performed after.
Similar content being viewed by others
Data availability
All data are available upon request.
References
de Souza PVS, de Rezende Pinto WBV, de Rezende Batistella GN, Bortholin T, Oliveira ASB (2017) Hereditary Spastic Paraplegia: Clinical and Genetic Hallmarks. Cerebellum 16(2):525–551
Ruano L, Melo C, Silva MC, Coutinho P (2014) The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology 42(3):174–183
Schüle R, Wiethoff S, Martus P et al (2016) Hereditary spastic paraplegia: Clinicogenetic lessons from 608 patients. Ann Neurol 79(4):646–658
Murala S, Nagarajan E, Bollu PC (2021) Hereditary spastic paraplegia. Neurol Sci 42(3):883–894
Darios F, Coarelli G, Durr A (2022) Genetics in hereditary spastic paraplegias: Essential but not enough. Curr Opin Neurobiol 72:8–14
Meyyazhagan A, Kuchi Bhotla H, Pappuswamy M, Orlacchio A (2022) The Puzzle of Hereditary Spastic Paraplegia: From Epidemiology to Treatment. Int J Mol Sci 23(14):7665 (Published 2022 Jul 11)
Iqbal Z, Rydning SL, Wedding IM et al (2017) Correction: Targeted high throughput sequencing in hereditary ataxia and spastic paraplegia. PLoS One 12(10):e0186571 (Published 2017 Oct 12)
Méreaux JL, Banneau G, Papin M et al (2022) Clinical and genetic spectra of 1550 index patients with hereditary spastic paraplegia. Brain 145(3):1029–1037
Perić S, Marković V, Candayan A et al (2022) Phenotypic and Genetic Heterogeneity of Adult Patients with Hereditary Spastic Paraplegia from Serbia. Cells 11(18):2804 (Published 2022 Sep 8)
Hedera P (2000) Hereditary Spastic Paraplegia Overview. In: Adam MP, Feldman J, Mirzaa GM et al (eds) GeneReviews®. University of Washington, Seattle, Seattle (WA)
Seo GH, Kim T, Choi IH et al (2020) Diagnostic yield and clinical utility of whole exome sequencing using an automated variant prioritization system, EVIDENCE. Clin Genet 98(6):562–570
Karczewski KJ, Francioli LC, Tiao G et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 581(7809):434–443 ([published correction appears in Nature. 2021 Feb;590(7846):E53] [published correction appears in Nature. 2021 Sep;597(7874):E3-E4])
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424
Köhler S, Gargano M, Matentzoglu N et al (2021) The Human Phenotype Ontology in 2021. Nucleic Acids Res 49(D1):D1207–D1217
Köhler S, Schulz MH, Krawitz P et al (2009) Clinical diagnostics in human genetics with semantic similarity searches in ontologies. Am J Hum Genet 85(4):457–464
Greene D, BioResource NIHR, Richardson S, Turro E (2016) Phenotype Similarity Regression for Identifying the Genetic Determinants of Rare Diseases. Am J Hum Genet 98(3):490–499. https://doi.org/10.1016/j.ajhg.2016.01.008
D’Amore A, Tessa A, Casali C et al (2018) Next Generation Molecular Diagnosis of Hereditary Spastic Paraplegias: An Italian Cross-Sectional Study. Front Neurol 9:981 (Published 2018 Dec 4)
Cui F, Sun L, Qiao J et al (2020) Genetic mutation analysis of hereditary spastic paraplegia: A retrospective study. Medicine (Baltimore) 99(23):e20193
Valencia CA, Husami A, Holle J et al (2015) Clinical Impact and Cost-Effectiveness of Whole Exome Sequencing as a Diagnostic Tool: A Pediatric Center’s Experience. Front Pediatr 3:67 (Published 2015 Aug 3)
Zhao M, Chen YJ, Wang MW et al (2019) Genetic and Clinical Profile of Chinese Patients with Autosomal Dominant Spastic Paraplegia. Mol Diagn Ther 23(6):781–789
Schiavoni S, Spagnoli C, Rizzi S et al (2020) Paediatric-onset hereditary spastic paraplegias: a retrospective cohort study. Dev Med Child Neurol 62(9):1068–1074
Mandelker D, Schmidt RJ, Ankala A et al (2016) Navigating highly homologous genes in a molecular diagnostic setting: a resource for clinical next-generation sequencing. Genet Med 18(12):1282–1289
Cummings BB, Marshall JL, Tukiainen T et al (2017) Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med 9(386):eaal5209
Kremer LS, Bader DM, Mertes C et al (2017) Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun 8:15824
Frésard L, Smail C, Ferraro NM et al (2019) Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat Med 25(6):911–919
Lee H, Huang AY, Wang LK et al (2020) Diagnostic utility of transcriptome sequencing for rare Mendelian diseases. Genet Med 22(3):490–499
Logsdon GA, Vollger MR, Eichler EE (2020) Long-read human genome sequencing and its applications. Nat Rev Genet 21(10):597–614
Yang JO, Yoon JY, Sung DH et al (2021) The emerging genetic diversity of hereditary spastic paraplegia in Korean patients. Genomics 113(6):4136–4148
Fussiger H, Pereira BLDS, Padilha JPD et al (2023) Copy number variations in SPAST and ATL1 are rare among Brazilians. Clin Genet 103(5):580–584
Drousiotou A, DiMeo I, Mineri R, Georgiou T, Stylianidou G, Tiranti V (2011) Ethylmalonic encephalopathy: application of improved biochemical and molecular diagnostic approaches. Clin Genet 79(4):385–390
Kitzler TM, Gupta IR, Osterman B et al (2019) Acute and Chronic Management in an Atypical Case of Ethylmalonic Encephalopathy. JIMD Rep 45:57–63
Pigeon N, Campeau PM, Cyr D, Lemieux B, Clarke JT (2009) Clinical heterogeneity in ethylmalonic encephalopathy. J Child Neurol 24(8):991–996
Müller vom Hagen J, Karle KN, Schüle R, Krägeloh-Mann I, Schöls L (2014) Leukodystrophies underlying cryptic spastic paraparesis: frequency and phenotype in 76 patients. Eur J Neurol 21(7):983–988
Hershkovitz E, Narkis G, Shorer Z et al (2002) Cerebral X-linked adrenoleukodystrophy in a girl with Xq27-Ter deletion. Ann Neurol 52(2):234–237
Jung HH, Wimplinger I, Jung S, Landau K, Gal A, Heppner FL (2007) Phenotypes of female adrenoleukodystrophy. Neurology 68(12):960–961
Moser HW (1997) Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120(Pt 8):1485–1508
Engelen M, van Ballegoij WJC, Mallack EJ et al (2022) International Recommendations for the Diagnosis and Management of Patients With Adrenoleukodystrophy: A Consensus-Based Approach. Neurology 99(21):940–951
Olgiati S, Doğu O, Tufekcioglu Z et al (2017) The p.Thr11Met mutation in c19orf12 is frequent among adult Turkish patients with MPAN. Parkinsonism Relat Disord. 39:64–70
Fraser S, Koenig M, Farach L, Mancias P, Mowrey K (2021) A De Novo case of autosomal dominant mitochondrial membrane protein-associated neurodegeneration. Mol Genet Genomic Med 9(7):e1706
Rickman OJ, Salter CG, Gunning AC et al (2021) Dominant mitochondrial membrane protein-associated neurodegeneration (MPAN) variants cluster within a specific C19orf12 isoform. Parkinsonism Relat Disord 82:84–86
Hartig MB, Iuso A, Haack T et al (2011) Absence of an orphan mitochondrial protein, c19orf12, causes a distinct clinical subtype of neurodegeneration with brain iron accumulation. Am J Hum Genet 89(4):543–550
Hogarth P, Gregory A, Kruer MC et al (2013) New NBIA subtype: genetic, clinical, pathologic, and radiographic features of MPAN. Neurology 80(3):268–275
Schulte EC, Claussen MC, Jochim A et al (2013) Mitochondrial membrane protein associated neurodegenration: a novel variant of neurodegeneration with brain iron accumulation. Mov Disord 28(2):224–227
Selikhova M, Fedotova E, Wiethoff S et al (2017) A 30-year history of MPAN case from Russia. Clin Neurol Neurosurg 159:111–113
Sparber P, Marakhonov A, Filatova A, Sharkova I, Skoblov M (2018) Novel case of neurodegeneration with brain iron accumulation 4 (NBIA4) caused by a pathogenic variant affecting splicing. Neurogenetics 19(4):257–260
Gregory A, Lotia M, Jeong SY et al (2019) Autosomal dominant mitochondrial membrane protein-associated neurodegeneration (MPAN). Mol Genet Genomic Med 7(7):e00736
Gregory A, Klopstock T, Kmiec T, Hogarth P, Hayflick SJ (2014) Mitochondrial Membrane Protein-Associated Neurodegeneration. In: Adam MP, Feldman J, Mirzaa GM et al (eds) GeneReviews®. University of Washington, Seattle, Seattle (WA)
Sparber P, Krylova T, Repina S et al (2021) Retrospective analysis of 17 patients with mitochondrial membrane protein-associated neurodegeneration diagnosed in Russia. Parkinsonism Relat Disord 84:98–104
Dursun U, Koroglu C, Kocasoy Orhan E, Ugur SA, Tolun A (2009) Autosomal recessive spastic paraplegia (SPG45) with mental retardation maps to 10q24.3–q25.1. Neurogenetics 10(4):325–331
Novarino G, Fenstermaker AG, Zaki MS et al (2014) Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 343(6170):506–511
Elsaid MF, Ibrahim K, Chalhoub N, Elsotouhy A, El Mudehki N, Abdel Aleem A (2017) NT5C2 novel splicing variant expands the phenotypic spectrum of Spastic Paraplegia (SPG45): case report of a new member of thin corpus callosum SPG-Subgroup. BMC Med Genet 18(1):33 (Published 2017 Mar 21)
Shenoy VS, Sampath R (2023) Syringomyelia. In: statpearls. Treasure island (FL): statpearls publishing. https://www.ncbi.nlm.nih.gov/books/NBK537110/. Accessed 10 Apr 2023
Arnoldi A, Crimella C, Tenderini E et al (2012) Clinical phenotype variability in patients with hereditary spastic paraplegia type 5 associated with CYP7B1 mutations. Clin Genet 81(2):150–157
Theuriet J, Pegat A, Leblanc P et al (2021) Phenoconversion from Spastic Paraplegia to ALS/FTD Associated with CYP7B1 Compound Heterozygous Mutations. Genes (Basel) 12(12):1876 (Published 2021 Nov 25)
Goizet C, Boukhris A, Durr A et al (2009) CYP7B1 mutations in pure and complex forms of hereditary spastic paraplegia type 5. Brain 132(Pt 6):1589–1600
Schlipf NA, Schüle R, Klimpe S et al (2011) Amplicon-based high-throughput pooled sequencing identifies mutations in CYP7B1 and SPG7 in sporadic spastic paraplegia patients. Clin Genet 80(2):148–160
Kumar KR, Blair NF, Vandebona H et al (2013) Targeted next generation sequencing in SPAST-negative hereditary spastic paraplegia. J Neurol 260(10):2516–2522
Roos P, Svenstrup K, Danielsen ER, Thomsen C, Nielsen JE (2014) CYP7B1: novel mutations and magnetic resonance spectroscopy abnormalities in hereditary spastic paraplegia type 5A. Acta Neurol Scand 129(5):330–334
Goizet C, Boukhris A, Maltete D et al (2009) SPG15 is the second most common cause of hereditary spastic paraplegia with thin corpus callosum. Neurology 73(14):1111–1119
Acknowledgements
Whole exome sequencing, analysis and variant interpretation were performed at the 3billion, Inc, Seoul, Republic of Korea as a part of 3billion's research funding program granted to DSP, VRS and SP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Board of the University Clinical Center of Serbia.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brankovic, M., Ivanovic, V., Basta, I. et al. Whole exome sequencing in Serbian patients with hereditary spastic paraplegia. Neurogenetics (2024). https://doi.org/10.1007/s10048-024-00755-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10048-024-00755-x