Abstract
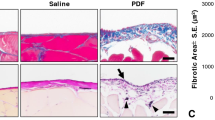
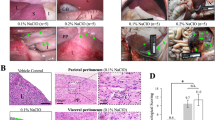
Peritoneal fluid dwell impacts the peritoneum by creating an abnormal physiological microenvironment. Little is known about the precise effects of fluid dwell on the peritoneum, and no adequate in vitro models to analyze the impact of fluid dwell have been established. In this study, we developed a peritoneal fluid dwell model combined with an artificial peritoneal cavity and fluid stirring generation system to clarify the effects of different dwelling solutions on the peritoneum over time. To replicate the peritoneal cavity, we devised a reconstructed peritoneal cavity utilizing a mesothelial layer, endothelial layer, and collagen membrane chamber. The reconstructed peritoneal cavity was infused with Dulbecco’s modified Eagle’s medium, saline, lactated Ringer’s solution or peritoneal dialysis solution with repeated 4-h dwells for 10 or 20 consecutive days. The above-described solutions induced epithelial–mesenchymal transition (EMT) and hyperplasia of mesothelial cells. All solution types modulated nitric oxide synthase activities in mesothelial and endothelial cells and nitric oxide concentrations in dwelling solutions. Inhibition of nitric oxide synthase activity acted synergistically on mesothelial EMT and hyperplasia. The present findings suggest that solutions infused into the peritoneal cavity are likely to affect nitric oxide production in the peritoneum and promote peritoneal fibrosis. Our newly devised peritoneal cavity model should be a promising tool for understanding peritoneal cellular kinetics and homeostasis.




Similar content being viewed by others
References
García-López E, Lindholm B, Davies S. An update on peritoneal dialysis solutions. Nat Rev Nephrol. 2012;8:224–33.
Yamaguchi H, Kojima H, Takezawa T. Vitrigel-eye irritancy test method using HCE-T cells. Toxicol Sci. 2013;135:347–55.
Aoki S, Makino J, Nagashima A, Takezawa T, Nomoto N, Uchihashi K, et al. Fluid flow stress affects peritoneal cell kinetics: possible pathogenesis of peritoneal fibrosis. Perit Dial Int. 2011;31:466–76.
Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, et al. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:227–34.
Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci USA. 2001;98:6476–81.
Combet S, Miyata T, Moulin P, Pouthier D, Goffin E, Devuyst O. Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol. 2000;11:717–28.
Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37.
Ni J, Moulin P, Gianello P, Feron O, Balligand J-L, Devuyst O. Mice that lack endothelial nitric oxide synthase are protected against functional and structural modifications induced by acute peritonitis. J Am Soc Nephrol. 2003;14:3205–16.
Chen J-Y, Chiu J-H, Chen H-L, Chen T-W, Yang W-C, Yang A-H. Human peritoneal mesothelial cells produce nitric oxide: induction by cytokines. Perit Dial Int. 2000;20:772–7.
Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int. 2004;66:1257–65.
White R, Barefield D, Ram S, Work J. Peritoneal dialysis solutions reverse the hemodynamic effects of nitric oxide synthesis inhibitors. Kidney Int. 1995;48:1986–93.
Reimann D, Dachs D, Meye C, Gross P. Amino acid-based peritoneal dialysis solution stimulates mesothelial nitric oxide production. Perit Dial Int. 2004;24:378–84.
Aoki S, Takezawa T, Oshikata-Miyazaki A, Ikeda S, Kuroyama H, Chimuro T, et al. Epithelial-to-mesenchymal transition and slit function of mesothelial cells are regulated by the cross talk between mesothelial cells and endothelial cells. Am J Physiol Renal Physiol. 2014;306:F116–22.
Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–13.
Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, Jimenez-Heffernan JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–13.
Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 2010;21:1077–85.
Kadoya H, Satoh M, Nagasu H, Sasaki T, Kashihara N. Deficiency of endothelial nitric oxide signaling pathway exacerbates peritoneal fibrosis in mice. Clin Exp Nephrol. 2014;. doi:10.1007/s10157-014-1029-3.
Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–19.
Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–9.
Devuyst O. New insights in the molecular mechanisms regulating peritoneal permeability. Nephrol Dial Transplant. 2002;17:548–51.
Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998;273:34724–9.
Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–508.
Polubinska A, Breborowicz A, Staniszewski R, Oreopoulos DG. Normal saline induces oxidative stress in peritoneal mesothelial cells. J Pediatr Surg. 2008;43:1821–6.
Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4.
Noh H, Kim J, Han K, Lee G, Song J, Chung S, et al. Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int. 2006;69:2022–8.
Cooke M, John P, Dzau M, Victor J. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509.
Witowski J, Topley N, Jorres A, Liberek T, Coles GA, Williams JD. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int. 1995;47:282–93.
Topley N, Kaur D, Petersen MM, Jorres A, Passlick-Deetjen J, Coles GA, et al. Biocompatibility of bicarbonate buffered peritoneal dialysis fluids: influence on mesothelial cell and neutrophil function. Kidney Int. 1996;49:1447–56.
Ogata S, Naito T, Yorioka N, Kiribayashi K, Kuratsune M, Kohno N. Effect of lactate and bicarbonate on human peritoneal mesothelial cells, fibroblasts and vascular endothelial cells, and the role of basic fibroblast growth factor. Nephrol Dial Transplant. 2004;19:2831–7.
Acknowledgments
We thank H. Ideguchi, M. Nishida, F. Mutoh, S. Nakahara, T. Takezawa (Teikyo University), and I. Nanbu for excellent technical assistance. We are grateful to Mr. K. Tokaichi for refining the English of the manuscript. This work was supported in part by an Agri-Health Translational Research Project (No. 6110) from the MAFF of Japan and JSPS KAKENHI Grant Number 25461701.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
H. Kuroyama and T. Chimuro are employees of Kanto Chemical Co. Inc. The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aoki, S., Noguchi, M., Takezawa, T. et al. Fluid dwell impact induces peritoneal fibrosis in the peritoneal cavity reconstructed in vitro. J Artif Organs 19, 87–96 (2016). https://doi.org/10.1007/s10047-015-0864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-015-0864-7