Abstract
Background
Neutral, low-glucose degradation product (GDP) peritoneal dialysis fluid (PDF) is less damaging to the peritoneum than conventional PDF but is still insufficient for biocompatibility. One remaining issue is the problem of buffering.
Methods
Using cultured rat peritoneal mesothelial cells (PMCs), the present study examined the difference between the effects of neutral low-GDP lactate PDF and neutral low-GDP bicarbonate/lactate PDF on cells. The effects of lactate stimulation on these cells were also examined.
Results
Lactate PDF enhanced mRNA expressions of α-smooth muscle actin (αSMA) and type 1 and type 3 collagens and lowered expression of e-cadherin mRNA in PMCs compared to bicarbonate/lactate PDF. Lactate stimulation increased mRNA expressions of αSMA, matrix metalloproteinase 2 (MMP2), and basic fibroblast growth factor (bFGF) and suppressed e-cadherin mRNA expression. Transforming growth factor (TGF)-β1 and TGF-β2 and collagen type 1 and 3 mRNA expressions were also enhanced by lactate stimulation.
Conclusions
These results suggest that lactate as a PDF buffer may act on PMCs to promote epithelial-mesenchymal transition (EMT) and production of TGF-β, bFGF, and collagen.
Similar content being viewed by others
Background
Peritoneal dialysis (PD) is an established treatment in renal replacement therapy, can be performed at home without bulky equipment, and can maintain a high quality of life. However, long-term PD treatment causes pathological changes to the peritoneum, such as detachment of mesothelial cells, fibrosis of the interstitium, increases in the new blood vessels, and narrowing of the vascular cavities [1]. These changes result in peritoneal dysfunction, making PD difficult to maintain. The risk of complications such as encapsulating peritoneal sclerosis due to peritoneal disorders is also increased [2]. To address this problem, a change has been made from acidic high-GDP PDF to neutral low-GDP PDF, achieving a reduction in rates of peritoneal disorders [3]. However, some problems remain with the biocompatibility of these new PDFs, with the issue of buffering representing one such problem. In Japan, all buffering agents for neutral low-GDP PDF to date have been lactate, but a bicarbonate/lactate buffer PDF has recently been released. However, few studies have examined the effects of buffering agents on the peritoneum. This study investigated the effects of neutral low-GDP PDF containing different buffers on the peritoneum and further clarified the effects of lactate on the peritoneum.
Methods
Dianeal N® (Baxter, Tokyo, Japan) was used as the lactate low-GDP PDF, and Reguneal® (Baxter) was used as the bicarbonate/lactate low-GDP PDF. Table 1 shows the constituents of these two PDFs.
Culture of PMCs
The isolation and culture of PMCs were performed according to our previously reported method [4]. Briefly, omentum was obtained from the abdominal cavity of Sprague-Dawley rats and dissolved in 0.05% trypsin and 0.02% ethylenediaminetetraacetate solution at 37 °C for 20 min. Cellular components were then isolated by centrifugation (150×g, 3 min) and washed with phosphate-buffered saline (PBS). Cells were suspended in Dulbecco’s modified Eagle’s medium/Ham’s Nutrient Mixture F12 (DMEM/F12) medium (Thermo Fisher Scientific, Yokohama, Japan) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Cromwell, CT), penicillin at 50 U/mL, and streptomycin at 50 μg/mL. Cells were seeded into 10-cm2 collagen type I-coated Petri dishes at 37 °C under a 5% CO2/95% air atmosphere. PMCs were identified by the uniform cobblestone appearance at confluence, by negative staining for von Willebrand factor, and by positive staining for both cytokeratin and vimentin. Confluent cultures at the third passage were used in experiments.
Examination of two different PDFs and two different buffers
Confluent cultured PMCs were divided into 5 groups: control group, L-PDF group, B/L-PDF group, L group, and B/L group. The control group used cultured mesothelial cells in DMEM/F12 + 10% FBS. The L-PDF and B/L-PDF groups used cultured cells in Dianeal N® or Reguneal® + 200 mM glutamate (Wako Pure Chemical Industries, Osaka, Japan) (both with the same content of DMEM/F12) + 10% FBS. The L and B/L groups used cultured cells in PBS + 40 mM lactate (Wako Pure Chemical Industries) or 30 mM NaHCO3 (Wako Pure Chemical Industries) with 10 mM lactate + 200 mM glutamate + 10% FBS. All culture media were adjusted to pH 7.0 using HCl. Cells were stimulated in these media for 72 h.
Examination of effects of lactate on PMCs
Confluent cultured PMCs were divided into 6 groups: control, L5, L10, L20, L40, and L80 groups. The control group used cultured mesothelial cells in PBS + 200 mM glutamate with 10% FBS. The L5, L10, L20, L40, and L80 groups used cultured cells in PBS + 200 mM glutamate with 10% FBS, with the addition of 5 mEq/L, 10 mEq/L, 20 mEq/L, 40 mEq/L, or 80 mEq/L of lactate, respectively. All culture media were adjusted to pH 7.0 using HCl. Cells were stimulated in these media for 6, 24, 48, and 72 h.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using NucleoSpin RNA (Macherey-Nagel, Düren, Germany) according to the instructions from the manufacturer. The cDNA was constructed with a SuperScript III for RT-PCR kit (Thermo Fisher Scientific, Yokohama, Japan). For amplification, qRT-PCR reaction solutions used 50 ng of cDNA, 10 μM of each primer, and 10 μL of FastStart Universal SYBR Green Master (Roche Molecular Diagnostics, Rotkreuz, Switzerland) in a volume of 38 μL with 30–35 cycles of 15 s at 95 °C and 15 s at 60 °C. Primer sequences are indicated in Table 2. The mRNA expression of each gene was represented by the ratio to GAPDH mRNA expression.
Statistical analysis
Data are presented as mean ± standard deviation. All statistical analyses were performed using the Kruskal-Wallis test, two-way analysis of variance, and Tukey’s honestly significant difference test on JMP Pro version 14.0 software (SAS Institute Japan, Tokyo, Japan). Values of P < 0.05 were considered significant.
Results
Effects of two different PDFs and buffers on PMCs
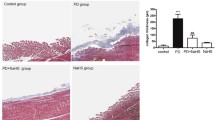
Expressions of mRNAs for αSMA and collagen types 1 and 3 were higher in the L-PDF group than in the control group. Expression of e-cadherin mRNA was lower in the L-PDF group than in the control group. However, in the B/L-PDF group, expressions of αSMA, e-cadherin, and collagen type 3 mRNAs did not differ significantly from those of controls. All gene expressions in the B/L PDF group were significantly different from those in the L-PDF group. Similarly, mRNA expressions of these genes in the L group were significantly different from those in the control group, but changes in mRNA expressions for the B/L group were small and differed from those in the L group (Fig. 1).
Examination of two different PDFs and two different buffers on PMCs. mRNA expression of αSMA (a), e-cadherin (b), collagen type 1 (c), and collagen type 3 (d). *P < 0.01 vs control, **P < 0.05 vs control,※P < 0.05 vs L-PDF, #P < 0.05 vs L-group, ##P < 0.01 vs L-group. In the L-PDF and L groups, αSMA, collagen type 1, and collagen type 3 mRNA expressions were enhanced and e-cadherin mRNA expression was decreased as compared with the control group. The B/L-PDF group and B/L group showed the same levels of expression of these genes as the control group
Effects of lactate on PMCs
Changes in each gene expression for the L5–L80 groups according to the stimulation time of 6–72 h are represented as the ratio to gene expression in the control group. Expressions of all genes showed significant interactions between the dose factor of lactate and the factor of stimulation time. Multiple differences were thus used to examine differences in stimulus time for each stimulus dose of 5–80 mEq/L and the difference in dose for each stimulus time (6–72 h). Gene expressions for each group are shown in Figs. 2, 3, and 4 for each stimulation time and each stimulation dose.
Effects of lactate on EMT of PMCs. Levels of mRNA expression for e-cadherin (a), αSMA (b), and MMP-2 (c) with each stimulus time:  control group,
control group,  L5 group,
L5 group,  L10 group,
L10 group,  L20 group,
L20 group,  L40 group,
L40 group,  L80 group, *P < 0.05 vs control group, **P < 0.01 vs control group. ***P < 0.001 vs control, #P < 0.0001 vs control group. Levels of mRNA expression for e-cadherin (d), αSMA (e), and MMP-2 (f) with each stimulus dose:
L80 group, *P < 0.05 vs control group, **P < 0.01 vs control group. ***P < 0.001 vs control, #P < 0.0001 vs control group. Levels of mRNA expression for e-cadherin (d), αSMA (e), and MMP-2 (f) with each stimulus dose:  6 h,
6 h,  24 h,
24 h,  48 h,
48 h,  72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. ***P < 0.001 vs 6 h. #P < 0.0001 vs 6 h. E-cadherin mRNA decreased (a, d) and αSMA mRNA expression increased (b, e) depending on lactate dose and stimulation time. MMP2 mRNA expression was stimulated at 48 and 72 h with 80 mEq/L (c, f)
72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. ***P < 0.001 vs 6 h. #P < 0.0001 vs 6 h. E-cadherin mRNA decreased (a, d) and αSMA mRNA expression increased (b, e) depending on lactate dose and stimulation time. MMP2 mRNA expression was stimulated at 48 and 72 h with 80 mEq/L (c, f)
Effects of lactate on TGF-β and bFGF mRNA expressions of PMCs. Levels of mRNA expression for TGF-β1 (a), TGF-β2 (b), and bFGF (c) for each stimulus time:  control group,
control group,  L5 group,
L5 group,  L10 group,
L10 group,  L20 group,
L20 group,  L40 group,
L40 group,  L80 group. **P < 0.01 vs control group. ***P < 0.001 vs control group.#P < 0.0001 vs control group. Levels of mRNA expression for TGF-β1 (d), TGF-β2 (e), and bFGF (f) with each stimulus dose:
L80 group. **P < 0.01 vs control group. ***P < 0.001 vs control group.#P < 0.0001 vs control group. Levels of mRNA expression for TGF-β1 (d), TGF-β2 (e), and bFGF (f) with each stimulus dose:  6 h,
6 h,  24 h,
24 h,  48 h,
48 h,  72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. ***P < 0.001 vs 6 h. #P < 0.0001 vs 6 h. TGFβ1 mRNA expression tended to be enhanced upon stimulation with 80 mEq/L (d). TGFβ2 mRNA expression was upregulated in a dose-dependent manner (e). Expression of bFGF mRNA was upregulated in a dose- and time-dependent manner (c, f)
72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. ***P < 0.001 vs 6 h. #P < 0.0001 vs 6 h. TGFβ1 mRNA expression tended to be enhanced upon stimulation with 80 mEq/L (d). TGFβ2 mRNA expression was upregulated in a dose-dependent manner (e). Expression of bFGF mRNA was upregulated in a dose- and time-dependent manner (c, f)
Effects of lactate on collagen genes. a Expressions of collagen type 1 mRNA for each stimulus time. b Expressions of collagen type 3 mRNA for each stimulus time  control group,
control group,  L5 group,
L5 group,  L10 group,
L10 group,  L20 group,
L20 group,  L40 group,
L40 group,  L80 group. **P < 0.01 vs control group. ***P < 0.001 vs control group. #P < 0.0001 vs control group. c Expression of collagen type 1 mRNA for each stimulus dose. d Expression of collagen type 3 mRNA for each stimulus dose:
L80 group. **P < 0.01 vs control group. ***P < 0.001 vs control group. #P < 0.0001 vs control group. c Expression of collagen type 1 mRNA for each stimulus dose. d Expression of collagen type 3 mRNA for each stimulus dose:  6 h,
6 h,  24 h,
24 h,  48 h,
48 h,  72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. #P < 0.0001 vs 6 h. Increased expression of collagen type 1 mRNA was observed in dose- and time-dependent manners (a, c). Increased expression of collagen type 3 mRNA was observed upon stimulation with lactate at 40 and 80 mEq/L (b, d)
72 h. *P < 0.05 vs 6 h. **P < 0.01 vs 6 h. #P < 0.0001 vs 6 h. Increased expression of collagen type 1 mRNA was observed in dose- and time-dependent manners (a, c). Increased expression of collagen type 3 mRNA was observed upon stimulation with lactate at 40 and 80 mEq/L (b, d)
Expression of e-cadherin mRNA decreased in a dose-dependent manner after 24, 48, and 72 h of stimulation with lactate (Fig. 2a). In the L20, L40, and L80 groups, mRNA expression decreased more with increasing stimulation time (Fig. 2d).
Dose- and time-dependent upregulations of αSMA mRNA expression were recognized after 48 and 72 h of stimulation (Fig. 2b, e). MMP2 mRNA expression tended to decrease with dose at 24, 48, and 72 h of stimulation, but increased with 80 mEq/L and 48 and 72 h of stimulation (Fig. 2c).
Expression of TGF-β1 mRNA was increased in the L80 group at 48 and 72 h after stimulation, but no increases were seen in the other groups (Fig. 3a). TGF-β2 mRNA expression tended to increase with increasing dose at 24 h stimulation, but no changes were seen for other stimulation times except with 80 mEq/L stimulation (Fig. 3b). In the L80 group, TGF-β2 mRNA expression was increased at 24 and 72 h after stimulation (Fig. 3e).
Expression of bFGF mRNA was accelerated in a dose-dependent manner (Fig. 3c) and increased in a time-dependent manner in the L80 group (Fig. 3f).
Expression of collagen type 1 and 3 mRNA also tended to increase dose- and time-dependently with lactate stimulation (Fig. 4).
Discussion
In the peritoneum of long-term PD patients, mesothelial cells degenerate and disappear, and interstitial fibrotic thickness and neovascularization have been reported [5]. These changes were affected by the PDF, and recognition of this fact led to the development of a biocompatible, neutral, low-GDP PDF. Many reports have shown that this new PDF results in less damage to the peritoneum than conventional acidic high-GDP PDF [3, 6, 7]. However, even this neutral, low-GDP PDF does not prevent all pathological changes to the peritoneum [8]. One of the causes is considered to be the effect of the buffer. Various reports have examined the effect of the buffer in PDF on the peritoneum, but most such studies have involved comparisons between conventional lactate PDF as a high-GDP PDF and neutral bicarbonate/lactate PDF as a low-GDP PDF [9,10,11]. Differences in the effects of these dialysates on the peritoneum, thus cannot be attributed to the buffer alone.
In Japan, neutral, low-GDP lactate PDF has been on the market for about 15 years, and all glucose dialysates have now been replaced with this solution. In addition to neutral, low-GDP lactate PDF, neutral low-GDP bicarbonate/lactate PDF was recently launched in Japan. The effect of the buffer in the PDF on the peritoneum was considered able to be examined by comparing these two PDFs. In the present study, the L-PDF group showed higher expression of αSMA and type 1 and 3 collagen mRNA and lower expression of e-cadherin mRNA in PMCs compared to the B/L-PDF PDF group. These phenomena were also observed in comparisons between the L-group and B/L group. Based on these results, lactate PDF may enhance EMT formation and collagen production by PMCs.
One report used the same two PDFs we used and found that bicarbonate/lactate PDF was less toxic to cell viability and apoptosis of PMCs than lactate PDF [12]. In addition, a clinical study [13] reported that Fibrin degradation products and vascular endothelial growth factor concentrations in effluent were lower when using bicarbonate/lactate PDF than when using lactate PDF. From our results and those reports, we consider that bicarbonate/lactate PDF may result in less peritoneal damage than lactate PDF.
We next examined the effects of lactate on EMT and collagen production in PMCs. Lactate decreased e-cadherin mRNA expression and increased αSMA mRNA expression in a dose- and time-dependent manner. MMP2 mRNA expression was increased with 80 mEq/L lactate at 48 and 72 h of stimulation. Increases in expressions of αSMA and MMP2 mRNAs and decreases in e-cadherin mRNA expression are phenomena occurring in EMT [14], suggesting that lactate may promote EMT in PMCs.
In this study, lactate at high doses promoted TGF-β1 and TGF-β2 mRNA expressions. In addition, bFGF mRNA expression was accelerated in dose- and time-dependent manners by lactate stimulation. Few studies have examined the effects of buffer alone on peritoneal cells [15, 16]. Ogata et al. [15] reported the effects of lactate, bicarbonate/lactate, and bicarbonate on human mesothelial cells, fibroblasts, and vascular endothelial cells. They showed that the highest amount of bFGF was produced by lactate, followed in order by bicarbonate/lactate and bicarbonate. They concluded that lactate acts on PMCs to increase bFGF production. Our results also showed that lactate increased expression of bFGF mRNA, similar to their results. TGF-β and bFGF are known facilitators of EMT [17]. Our results suggest that lactate may activate TGF-β and bFGF production and cause EMT.
Expressions of collagen type 1 and type 3 mRNAs were also stimulated in dose- and time-dependent manners by lactate. EMT is greatly involved in tissue fibrosis, and lactate may cause EMT in PMCs to increase collagen production and promote peritoneal fibrosis.
Many papers have described lactate produced by lactate dehydrogenase as triggering EMT in several cancer cells [18,19,20]. Several studies have also examined the relationships between lactate, EMT, and fibrosis [21,22,23]. In those reports, lactate was considered to enhance the production of TGF-β, leading to enhanced EMT and collagen production. Among these studies, Kottmann et al. [21, 22] attributed the lactate-enhancing effects of EMT to the low pH, whereas Zareie et al. [24] showed that lactate PDF was associated with stronger angiogenesis and enhanced fibrosis regardless of pH. They concluded that the action of lactate was unrelated to pH. Our experiments standardized the pH at 7.0 to remove the need to account for the effects of pH. As a result, lactate was seen to increase EMT, TGF-β, bFGF, and collagen production. We therefore believe that these effects are unrelated to pH, with lactate itself leading to the promotion of EMT, promotion of collagen production, and peritoneal fibrosis. In this study, only mRNA expressions were measured as markers for the effects of lactate on EMT and collagen expressions, but measurement at the protein level is also necessary, and such studies are planned for the future. Signal pathways that cause EMT of TGFβ and bFGF have been known to involve smad2/3 and snail. We believe that further research about signal pathways including smad2/3 and snail is needed on this point.
In the experiment that changed the dose and timing of lactate and examined mRNA expressions of MMP2, TGFβ1, and collagen type 3, no significant increases were observed with 40 mEq/L lactate alone, but only at 80 mEq/L. However, changes in EMT and fibrotic mRNA were observed by stimulation with PDF containing 40 mEq/L lactate. We considered that these differences may be influenced by the GDP contained in PDF, as even neutral PDF contains GDP, albeit at low concentrations. We hope to investigate in closer detail the effects of 40 mEq/L lactate + GDP on mesothelial cells in the future.
In the mechanism of peritoneal fibrosis, mesothelial cells have been considered an important source of myofibroblasts through the EMT. However, Chen et al. [25] reported that submesothelial fibroblasts are major precursors of peritoneal myofibroblasts using an in vivo model. Lua et al. [26] have been pointed out that only 17% of myofibroblasts were derived from mesothelial cells in peritoneal fibrosis. The fibrogenic cells have not been identified with certainly. We think further consideration is needed in this regard.
Conclusions
Lactate PDF enhanced expressions of αSMA and collagen mRNA and lowered expression of e-cadherin mRNA in peritoneal mesothelial cells more strongly than lactate/bicarbonate PDF. Lactate showed an EMT-promoting effect and TGFβ, bFGF, and collagen production-enhancing effects on mesothelial cells. These results suggest that lactate in PDF may have harmful effects on PMCs.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- GDP:
-
Glucose degradation product
- PDF:
-
Peritoneal dialysis fluid
- PMCs:
-
Peritoneal mesothelial cells
- αSMA:
-
α-Smooth muscle actin
- MMP2:
-
Matrix metalloproteinase 2
- bFGF:
-
Basic fibroblast growth factor
- TGF-β:
-
Transforming growth factor-β
- EMT:
-
Epithelial mesenchymal transition
- PD:
-
Peritoneal dialysis
- PBS:
-
Phosphate-buffered saline
- DMEM/F120:
-
Dulbecco’s modified Eagle’s medium/Ham’s Nutrient Mixture F12
- FBS:
-
Fetal bovine serum
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–9.
Nakayama M, Miyazaki M, Honda K, Kasai K, Tomo T, Nakamoto H, et al. Encapsulating peritoneal sclerosis in the ERA of multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit Dial Int. 2014;34:766–74.
Kawanishi K, Honda K, Tsukada M, Oda H, Nitta K. Neutral solution low in glucose degradation products is associated with less peritoneal fibrosis and vascular sclerosis in patients receiving peritoneal dialysis. Perit Dial Int. 2013;33:242–51.
Higuchi C, Tanihata Y, Nishimura H, Naito T, Sanaka T. Effect of glucose and plasminogen activator inhibitor-1 on collagen metabolism in the peritoneum. Ther Apher Dial. 2005;9:173–81.
Alston H, Fan S, Nakayama M. Encapsulating peritoneal sclerosis. Semin Nephrol. 2017;37:93–102.
Tawada M, Hamada C, Suzuki Y, Sakata F, Sun T, Kinashi H, et al. Effects of long-term treatment with low-GDP, pH-neutral solutions on peritoneal membranes in peritoneal dialysis patients. Clin Exp Nephrol. 2019;23:689–99.
Witowski J, Korybalska K, Ksiazek K, Wisniewska-Elnur J, Jörres A, Lage C, et al. Peritoneal dialysis with solutions low in glucose degradation products is associated with improved biocompatibility profile towards peritoneal mesothelial cells. Nephrol Dial Transplant. 2004;19:917–24.
Hamada C, Honda K, Kawanishi K, Nakamoto H, Ito Y, Sakurada T, et al. Morphological characteristics in peritoneum in patients with neutral peritoneal dialysis solution. J Artif Organs. 2015;18:243–5.
Park MS, Kim JK, Holmes C, Weiss MF. Effects of bicarbonate/lactate solution on peritoneal advanced glycation end-product accumulation. Perit Dial Int. 2000;20(Suppl 5):S33–8.
Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat models. Kidney Int. 2005;67:1559–65.
Vila CM, Keuning ED, Talhout W, Paauw NJ, van Ittersum FJ, ter Wee PM, et al. Differences in peritoneal response after exposure to low-GDP bicarbonate/lactate-buffered dialysis solution compared to conventional dialysis solution in a uremic mouse model. Int Urol Nephrol. 2018;50:1151–61.
Kuma A, Tamura M, Ishimatsu N, Harada Y, Izumi H, Miyamoto T, et al. Monocarboxylate transporter-1 mediates the protective effects of neutral-pH bicarbonate/lactate-buffered peritoneal dialysis fluid on cell viability and apoptosis. Ther Apher Dial. 2017;21:62–70.
Hoshino T, Ishii H, Kitano T, Shindo M, Miyazawa H, Yamada H, et al. Effects of a new bicarbonate/lactate-buffered neutral peritoneal dialysis fluid for peritoneal failure in patients undergoing peritoneal dialysis. Discovery Medicine. 2016;21:81–8.
Morandi A, Taddei ML, Chiarugi P, Giannoni E. Targeting the metabolic reprogramming that controls epithelial-to-mesenchymal transition in aggressive tumors. Front Oncol. 2017;7:40. https://doi.org/10.3389/fonc.2017.00040.
Ogata S, Naito T, Yorioka N, Kiribayashi K, Kuratsune M, Kohno N. Effect of lactate and bicarbonate on human peritoneal mesothelial cells, fibroblasts and vascular endothelial cells, and the role of basic fibroblast growth factor. Nephrol Dial Transplant. 2004;19:2831–7.
Plum J, Razeghi P, Lordnejad RM, Perniok P, Fleisch M, Fuβhõller A, et al. Peritoneal dialysis fluids with a physiologic pH based on either lactate or bicarbonate buffer-effects on human mesothelial cells. Am J Kidney Dis. 2001;38:867–75.
Strutz F, Zeiberg M, Ziyadeh FN, Yang C-Q, Kalluri R, Muller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–28.
Zhao J, Huang X, Xu Z, Dai J, He H, Zhu Y, et al. LDHA promotes tumor metastasis by facilitating epithelial-mesenchymal transition in renal carcinoma. Mol Med Rep. 2017;16:8335–44.
Liu M, Quek LE, Sultani G, Turner N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 2016;4:19. https://doi.org/10.1186/s40170-016-0160-x.
Cai H, Li J, Zhang Y, Liao Y, Zhu Y, Wang C, et al. LDHA promotes oral squamous cell carcinoma progression through facilitating glycolysis and epithelial-mesenchymal transition. Front Oncol. 2019;9:1446 (doi: https://doi.org/10.3389/fonc.2019.01446. eCollection 2019).
Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med. 2012;186:740–51.
Kottmann RM, Trawick E, Judge JL, Wahl LA, Epa AP, Owens KM, et al. Pharmacologic inhibition of lactate production prevents myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1305–12.
Yalamanchi N, Klein MB, Pham HM, Longaker MT, Chang J. Flexor tendon wound healing in vitro: lactate up-regulation of TGF-beta expression and functional activity. Plast Reconstr Surg. 2004;113:625–32.
Zareie M, Keuning ED, ter Wee PM, Schalkwijk CG, Beelen RHJ, van den Born J. Improved biocompatibility of bicarbonate/lactate-buffered PDF is not related to pH. Nephrol Dial Transplant. 2006;21:208–16.
Chen Y-T, Chang Y-T, Pan S-Y, Chou T-H, Chang F-C, Yeh P-Y, et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. L Am Soc Nephrol. 2014;25:2847–58.
Lua I, Li Y, Pappoe LS, Asahina K. Myofibroblastic conversion and regeneration of mesothelial cells in peritoneal and liver fibrosis. Am J Pathol. 2015;185:3258–73.
Acknowledgements
Not applicable in this section.
Funding
All research funds were provided by the Tokyo Women’s Medical University research funds.
Author information
Authors and Affiliations
Contributions
CH made substantial contributions to the study conception and design and performed the experiment, acquisition of the data, analysis, and interpretation of the data. JK performed the entire experiment. HS helped to draft the manuscript. The authors have read and approved the final manuscript submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were conducted in compliance with the protocol, which was reviewed by the Institutional Animal Care and Use Committee and approved by the Ethics Committee of Tokyo Women’s Medical University (Permit Numbers: AE-18-039, AE-19-030).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Higuchi, C., Kuriyama, J. & Sakura, H. Effect of lactate as a peritoneal dialysis fluid buffer on rat peritoneal mesothelial cells. Ren Replace Ther 6, 61 (2020). https://doi.org/10.1186/s41100-020-00306-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-020-00306-8