Abstract
Although acute pulmonary injury after cardiopulmonary bypass has been detailed in the literature, it was seldom mentioned in the context of following implantation of a ventricular assist device. We report on a 65-year-old male with end-stage ischemic cardiomyopathy who underwent implantation of Levitronix CentriMag (Levitronix, Waltham, MA) for cardiac support and was listed for heart transplantation. Acute pulmonary injury with profound hypoxaemia was noted 6 h after the implantation. Despite optimal medical treatment and maximal ventilator support, refractory hypoxaemia persisted, and veno-venous extracorporeal membrane oxygenation (oxygenator: Affinity-NT; centrifugal pump: BPX-80 Bio-Pump, Medtronic, Minneapolis, MN, USA) was applied for ventilation support. The patient was weaned from the extracorporeal membrane oxygenation 4 days later and from the ventilator on the next 2 days. He underwent a successful orthotopic heart transplant after a total of 77 days on Levitronix left ventricular device cardiac support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventricular assist devices (VADs) are generally used for patients with advanced heart failure. However, they can, indeed, bring about complications, especially thromboembolism and bleeding tendency [1, 2]. Although acute pulmonary injury is common after extracorporeal circulation [3], it is seldom reported on as post-VAD implantation. Veno-venous extracorporeal membrane oxygenation (VV ECMO) is the ultimate treatment for acute pulmonary injury with profound hypoxaemia [4] if medical treatment fails. We report a rare case of successful application of VV ECMO for acute pulmonary injury after implantation of Levitronix CentriMag VAD.
Case report
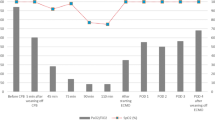
A 65-year-old man was sent to our emergency room due to angina pectoris and cold sweating. ST-elevation myocardial infarction was present on electrocardiography, and the troponin-I level was up to 66.6 ng/ml. Emergent coronary artery angiogram demonstrated acute occlusion of triple vessels and the need for emergent on-pump coronary artery bypass graft (CABG) surgery, wherein total revascularization was done (vein graft to left anterior descending, obtuse marginal, and distal right coronary arteries). After the CABG, low cardiac output was noted, and the hemodynamic status could be reluctantly maintained with inotropic agents and an intra-aortic balloon pump (IABP). One week later, the patient could be weaned from the ventilator, but couldn’t be weaned from the IABP despite optimal medical treatment. The echocardiography showed poor contractility of the left ventricle (ejection fraction: 15–20 %). He was transferred to the ordinary ward and was listed for urgent cardiac transplantation. But low cardiac output syndromes were still present, including low blood pressure, deteriorated renal function with progressive oligouria, and flatulence. Due to his critical condition, we implanted a continuous-flow Levitronix CentriMag VAD (Levitronix, Waltham, MA) for advanced left ventricular support. Via re-sternotomy and under guidance of trans-esophageal echocardiography (TEE), the venous drainage tube was inserted from the right superior pulmonary vein into the left ventricular apex (Fig. 1a) while the arterial tube was cannulated on the ascending aorta. With extracorporeal circulation standby, the total operation time was nearly 2 h. Total blood loss during the operation was less than 50 ml. No blood transfusion was needed, and the hematocrit level was about 36 %. The systolic function of the right ventricle appeared preserved on low-dose inotropic support. The Levitronix CentriMag VAD could provide 3.5 l flow per minute with 3000 revolutions per minute. The Swan-Ganz catheter showed the pulmonary capillary wedge pressure (PCWP) was 7–8 mmHg with VAD support. The PCWP was around 10–11 mmHg after resuscitation with 500 ml Voluven (Tetrastarch, 6 %) solution. The hemodynamic status was stable, and the IABP was removed. The patient was sent to the cardiac intensive care unit. According to continuous monitoring of the Swan-Ganz catheter, the central venous pressure was maintained at 12 mmHg, and the PCWP was maintained at 9–10 mmHg. The cardiac index was around 2.6–3.0 l/min per square meter. Little bloody drainage was noted from the mediastinum tube. The initial chest radiography showed increased markings and only mild infiltrations in both lower lungs and blunting of the bilateral costophrenic angles (Fig. 2a). The ventilator was set with positive end expiration pressure (PEEP) 6 cmH2O and support pressure 12 cmH2O. The analysis of arterial blood gas showed the partial pressure of oxygen (PaO2) was 140–150 mmHg, with 40 % fraction of inspiration O2 (FiO2). The patient was conscious and could obey instructions. But 6 h later, progressive desaturation appeared and the repeated chest radiography showed deteriorated change with multiple focal patchy alveolar consolidations and infiltrations in both lungs. (Fig. 2b) Despite maximal ventilator support, the PaO2 was only 58 mmHg under 100 % FiO2; the mean airway pressure was 30 cmH2O. The oxygenation index was 42, and the alveolar-arterial oxygen difference was 573 mmHg. The PCWP was 12 mmHg. The repeated TEE proved unloading of the left ventricle with the VAD support (Fig. 1b). Under the presumed diagnosis of acute respiratory distress syndrome, we applied a VV ECMO for the critical hypoxaemia. The ECMO circulation system contains a heparin-coated polypropylene oxygenator (Affinity, NT; Medtronic, Minneapolis, MN, USA) and a centrifugal pump (BPX-80 Bio-Pump, Medtronic, Minneapolis, MN, USA). The venous drainage tube was from the right femoral vein, and the arterial perfusion tube was into the right internal jugular vein. Systemic heparinization was done to keep active clotting time between 140 and 160 s. During the ECMO period, the ventilator was set with 40 % FiO2 and with PEEP 10–12 cmH2O to avoid alveolar collapse. The support pressure was set to 10–12 cmH2O to carry out a low tidal volume status (<6 ml/kg), and plateau pressure was under 30 cmH2O. With the support of VAD and ECMO, we did not apply prone positioning, inverse ratio ventilation, or high frequency ventilation. We used low-dose intravenous corticosteroids, oral NSAIDs, and Colchicine as anti-inflammatory therapy. Lasix was administered to keep the dry lung status, and the PCWP was continuously monitored under 8 mmHg via Swan-Ganz catheter. Daily trans-thoracic echocardiography was done to confirm the unloading of left ventricle, position of the cannula tip, and absence of intra-cardiac thrombus formation. The pulmonary parenchyma recovered well, and the ventilation and oxygenation improved. The patient was weaned from ECMO 4 days later and from the ventilator over the next 2 days. The chest radiography showed resolution of infiltration and good recovery of pulmonary parenchyma. The clinical time course is displayed in Table 1. No ECMO-related complication was noted. The VAD could not be tapered, and the patient underwent a successful orthotopic cardiac transplantation after 77 days on the Levitronix VAD. He was extubated on the next day after cardiac transplant and was discharged home 2 weeks later after a total hospital stay of 85 days. In the outpatient follow-up, there were no cardiac rejection or device-related complications noted.
a Radiography immediately after VAD implantation: increased markings and mild infiltrations over the bilateral lower lungs and blunting of the bilateral costophrenic angles. b Radiography 6 h after VAD implantation: multiple focal patchy alveolar consolidations and infiltrations over the bilateral lungs.
Discussion
In the literature, systemic inflammatory response syndrome (SIRS) has been observed after VAD implantation [5, 6]. However, acute pulmonary injury after VAD implantation was seldom reported. Inflammatory response caused by the mechanical device may be the major cause of acute pulmonary injury. The contact of blood with the non-endothelium artificial surface would activate the inflammatory system. The complex pathways involve the intrinsic and extrinsic coagulation system, the fibrinolysis system, the complement system, and the cellular immune system [7]. In most circumstances, the SIRS is transient and self-terminating because the human defense mechanism is able to compensate. However, sequela organ dysfunction might occur once the SIRS is out of control, which will increase intensive care and even mortality. In this case, we didn’t apply cardiopulmonary bypass or blood transfusion during VAD implantation. We thought the acute lung injury mainly resulted from the SIRS caused by the exposure of blood to a non-physiologic surface. The treatment for SIRS caused by a mechanical circuit includes pharmacological and technical strategies [8]. The pharmacological strategies focus on inhibition of inflammatory responses and include steroids, serine protease inhibitors (aprotinin), complement inhibitors, and phosphodiesterase inhibitors. The technical strategies include heparin-bonded circuits, ultrafiltration, and leukocyte filtration. As to the pulmonary injury, VV ECMO can provide proper systemic oxygenation if profound hypoxaemia could not be corrected via optimal medical therapy and maximal ventilator support [4]. With the aid of ECMO support, we waited until the lung recovered by itself because most cases of lung injury caused by SIRS are transient and self-cured under combinative pharmacological treatment.
In conclusion, acute pulmonary injury after VAD implantation is uncommon, but lethal if the profound hypoxaemia cannot be corrected. In addition to pharmacological strategies to inhibit the inflammatory response, ECMO could be applied in these critical conditions.
References
Fang JC. Rise of the machines-left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–5.
Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–33.
Asmakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1107–15.
Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–14.
Wilhelm CR, Ristich J, Kormos PL, Wagner WR. Monocyte tissue factor expression and ongoing complement generation in ventricular assist device patients. Ann Thorac Surg. 1998;65:1071–6.
Deng MC, Erren M, Tjan TD, Tamminga N, Werntze B, Zimmermann P, Weyand M, Hammel D, Schmidt C, Scheld HH. Left ventricular assist system support is associated with persistent inflammation and temporary immunosuppression. Thorac Cardiovasc Surg. 1999;47:326–31.
Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 1—mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009;23:223–31.
Warren OJ, Watret AL, de Wit KL, Alexiou C, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 2—anti-inflammatory therapeutic strategies. J Cardiothorac Vasc Anesth. 2009;23:384–93.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, JL., Sung, SY., Hsu, PS. et al. Acute pulmonary injury with refractory hypoxaemia after implantation of Levitronix CentriMag ventricular assist device: successful treatment with veno-venous extracorporeal membrane oxygenation. J Artif Organs 17, 202–205 (2014). https://doi.org/10.1007/s10047-013-0750-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-013-0750-0