Abstract
The automated identification of brain structure in Magnetic Resonance Imaging is very important both in neuroscience research and as a possible clinical diagnostic tool. In this study, a novel strategy for fully automated hippocampal segmentation in MRI is presented. It is based on a supervised algorithm, called RUSBoost, which combines data random undersampling with a boosting algorithm. RUSBoost is an algorithm specifically designed for imbalanced classification, suitable for large data sets because it uses random undersampling of the majority class. The RUSBoost performances were compared with those of ADABoost, Random Forest and the publicly available brain segmentation package, FreeSurfer. This study was conducted on a data set of 50 T1-weighted structural brain images. The RUSBoost-based segmentation tool achieved the best results with a Dice’s index of \(0.88 \pm 0.01\) (\(0.87 \pm 0.01\)) for the left (right) brain hemisphere. An independent data set of 50 T1-weighted structural brain scans was used for an independent validation of the fully trained strategies. Again the RUSBoost segmentations compared favorably with manual segmentations with the highest performances among the four tools. Moreover, the Pearson correlation coefficient between hippocampal volumes computed by manual and RUSBoost segmentations was 0.83 (0.82) for left (right) side, statistically significant, and higher than those computed by Adaboost, Random Forest and FreeSurfer. The proposed method may be suitable for accurate, robust and statistically significant segmentations of hippocampi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The role of neuroimaging in the study of brain disease and for clinical diagnostic purposes has acquired increasing importance. The possibility of investigating the morphology of specific brain structures relies on their accurate delimitation from the surrounding brain parenchyma and from the other adjacent structures (segmentation). This proves particularly challenging for structures characterized by morphological complexity, such as the hippocampus, a part of the temporal lobe with a prominent role in memory and other cognitive functions. The hippocampus is primarily involved in the pathogenesis of a number of conditions, firstly Alzheimer’s disease (AD), the most common type of dementia [1]. Nowadays, a definite diagnosis of AD can only be made if there is histopathological confirmation, either post-mortem or on brain biopsy. However, biomarkers of the disease supportive of the diagnosis are now recognized, and these include structural brain changes visible on Magnetic Resonance Images (MRIs), in particular atrophy of the medial temporal lobe and in particular of the hippocampal formation [2–7].
Manual segmentation of hippocampus has been so far considered the gold standard, despite the heterogeneity of anatomical landmarks and protocols adopted [8]; it is also laborious, time consuming and prone to rater error. Automated segmentation techniques are gaining increasing recognition since, not only they offer the possibility of studying rapidly large databases, for example in pharmaceutical trials or genetic research, but also afford higher test–retest reliability and the robust reproducibility needed for multi-centric studies. In the last few years, state-of-the-art hippocampal segmentation from 3D MRI research has delineated a few major approaches. Multi-atlas methods, among which the joint label fusion technique proposed by Wang et al. [9], are based on information propagation between multiple atlases, and bias correction. Other approaches are based on the active contour models (ACM) [10], in which a deformable contour is iteratively adapted to the image in order to generate the partition of the ROI. Machine learning approaches, on the contrary, use statistical tools from image processing techniques to perform the segmentation of the hippocampus, by focusing on the delineation of most characterizing features (texture, shape, edges). Among them, Morra et al. [11, 12] showed the validity of this approach for accurate segmentation of the hippocampal region. Hence, building accurate tools for the identification of brain structures in MRI is a promising approach to identify anatomical differences that can be associated with the presence or absence of neurodegenerative diseases, such as AD. The brain images mostly contain noise, inhomogeneity and sometime deviation [13], therefore accurate segmentation of brain images in a difficult task. Despite numerous efforts described in the literature [11, 14–19], segmentation is still commonly performed manually by experts.
The main goal of this work was to develop an accurate strategy based on supervised learning algorithms for hippocampal segmentation using 3D brain MRI. The task of a classifier, trained on a set of previously labeled examples (MR images in which the hippocampi had been previously manually segmented), is to classify voxels of a new brain MR image as belonging or not to the hippocampus. In this study, the performance of a novel statistical strategy, based on RUSBoost [20], was evaluated for hippocampal segmentation. RUSBoost was designed for imbalanced classification problems, combining data random undersampling with boosting. It is an alternative of another data sampling/boosting algorithm called SMOTEBoost [21] which uses an oversampling technique, creating new minority class examples by extrapolating between existing examples, combined with boosting technique. Creating new examples, SMOTEBoost increases model training times. It has been successful in applications [22, 23] where not too big data sets were analyzed. As the training data increases in size, the SMOTE run time increases, incurring the risk of becoming impractical. When a data set is very large, as for 3D MRI data sets, selecting an appropriate sampling method becomes important. Training a model on very large data set would take much less if undersampling is used as for RUSBoost. The drawback associated with undersampling is the loss of information that comes with deleting examples from the training data. Moreover, there is evidence that the RUSBoost algorithm performs favorably when compared to SMOTEBoost, while being a simpler and faster technique that often results in significantly better classification performance [21, 24]. To the best of our knowledge, this is the first application of RUSBoost classifiers to hippocampal segmentation.
This work utilizes two datasets, obtained from the Alzheimers Disease Neuroimaging Initiative (ADNI, http://adni.loni.usc.edu/) database, consisting of MR images and their corresponding expert manual labels produced with a standard harmonized protocol. The first data set, DB1, was used for training the algorithms and estimating evaluation metrics via cross validation. The RUSBoost performances on DB1 were excellent when compared with those of three classifiers, Adaboost [25], Random forest (RF) [26] and FreeSurfer v.5.1 [15]. Adaboost is a boosting algorithm that sequentially selects weak classifiers and weights each of them based on their error. It has been previously employed as segmentation tools in [11]. RF uses multiple binary decision trees, and recently several brain MRI segmentation systems based on RF classifiers have appeared in the literature [16, 19, 27–29]. FreeSurfer is a publicly available package and can be considered the state-of-the-art whole-brain segmentation tool, since numerous imaging studies across multiple centers have shown its robustness and accuracy [30].
The second data set, DB2, was employed for an assessment of the performance of the fully trained classifiers. Results on the DB2 data set confirmed those obtained in the previous analysis and showed that the RUSBoost segmentation strategy, trained on DB1, generalized very well on the independent data set, avoiding problems like overfitting. Moreover, the hippocampal volumes obtained with our RUSBoost segmentation showed the best correlation with those segmented manually, which is very important for diagnostic purposes.
For all the classifiers, we also evaluated how the Dice’s index varied with the training set size, providing practical guidelines for future users.
2 Materials and methods
2.1 Data set description
The data used in the preparation of this study were obtained from the Alzheimers Disease Neuroimaging Initiative (ADNI, http://adni.loni.usc.edu) database. The ADNI was launched in 2003 by the NIA, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the U.S. Food and Drug Administration (FDA), private pharmaceutical companies, and nonprofit organizations. For up-to-date information, see http://www.adni-info.org.
Two databases of T1-weighted whole-brain MR images, DB1 and DB2, were used in the study, both including normal controls (NC), subjects with mild cognitive impairment (MCI) and patients with Alzheimer’s disease (AD). All images were downloaded from the ADNI LONI Image Data Archive (https://ida.loni.usc.edu). Both DB1 and DB2 data sets consisted of 50 subjects each whose demographic details are reported in Table 1. All the images were acquired on 1.5 Tesla, and 3.0 Tesla scanners which specifications are reported in Table 2.
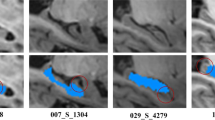
Bilateral hippocampi were manually segmented using the Harmonized Hippocampal Protocol (http://www.hippocampal-protocol.net/) [31, 32] which aims to standardize the available manual segmentation protocols. The more inclusive definition of the Harmonized protocol may also limit the inconsistencies due to the use of arbitrary lines and tissue exclusion of the currently available manual segmentation protocols.
Preprocessing involved a first registration through a six-parameter affine transformation to the Montreal Neurological Institute MNI152 template. Then a gross peri-hippocampal volume was extracted for left and right hippocampi for each scan and for the template; these regions underwent a further affine registration using the template hippocampal boxes as reference images. In this way, two Volumes of Interest (VOIs) of dimension \(50 \times 60 \times 60\) were obtained. The two registrations and box extraction were fully automated.
2.2 Features
The 3D segmentation was performed using for each voxel a vector of 315 elements (Table 1) representing information about position, intensity, neighboring texture, and local filters. Haar-like and Haralick features provide information on image texture, in particular on contrast, uniformity, rugosity, regularity, etc. [33–36]. A number of 248 Haar-like features were calculated spanning a 3D filter of varying dimensions (from \(3 \times 3 \times 3\) to \(9 \times 9 \times 9\)) for each voxel and averaging the voxels intensities in each VOI. Forty-eight Haralick features were calculated; in particular energy, contrast, correlation and inverse difference moment were computed based on the calculation of gray level co-occurrence matrix (GLCM), created on the \(n \times n\) voxels (n varying from 3 to 9) projection subimages of the volume centered in each voxel. A study on local Haralik features has been previously carried out showing their successful application to hippocampal segmentation [27]. Finally, the gradients calculated in different directions and at different distances, and the relative positions of the voxels (x, y, z) were included as additional features.
2.3 RUSBoost
RUSBoost is a boosting-based sampling algorithm designed to handle class imbalance. It combines Random UnderSampling (RUS) and Adaboost. RUS is a technique that randomly removes examples from the majority class until the desired balance is achieved. Let \(x _i\) be a point in the feature space X and \(y_i\) be a class label in \(Y=\{-1,+1\}\). The data set S can be represented by the tuple \(( x _i, y_i)\) with \(i=1, 2,\ldots , m\). The algorithm assigns to each example the weight \(D_1(i)=\frac{1}{m}\) for \(i=1, 2,\ldots , m\). Then, in each round \(t=1, 2 \ldots , T\), the following steps are performed.
-
1.
A temporary training set \(S_t'\) is created with distribution \(D_t'\) using random undersampling (RUS). It is applied to remove the majority class examples until the percentage N of \(S_t'\) belongs to the minority class.
-
2.
A weak learner is called providing it with examples \(S_t'\) and their weights \(D_t'\).
-
3.
A hypothesis \(h_t: X \times Y \rightarrow [0,1]\), which associates to every example \(x_i\) the probability to get the correct label \(y_i\) or the incorrect label \(y_i\), is obtained. If \(h_t(x_i, y_i) = 1\) and \(h_t(x_i, y : y \ne y_i) = 0\) then \(h_t\) has correctly predicted that the \(x_i\)’s label is \(y_i\), not y. Similarly, if \(h_t(x_i, y_i) = 0\) and \(h_t(x_i, y : y \ne y_i) = 1\), \(h_t\) has incorrectly predicted that the \(x_i\)’s labels is y.
-
4.
The pseudo-loss for S and \(D_t\) is calculated:
$$\begin{aligned} \epsilon _t=\sum _{(i,y):y_i\ne y} D_t(i) (1-h_t(x_i,y_i)+h_t(x_i,y)) \end{aligned}$$It is a modified version of Adaboost error function: here an higher cost is assigned to the examples with higher probability of being misclassified by the weak learner.
-
5.
The weight update parameter is calculated:
$$\begin{aligned} \alpha _t=\frac{\epsilon _t}{1-\epsilon _t} \end{aligned}$$For \(\epsilon _t \le \frac{1}{2}\), \(\alpha _t \le 1\).
-
6.
Update \(D_t\):
$$\begin{aligned} D_{t+1}(i)\,=\, & {} D_t(i)\alpha _t^{\frac{1}{2}(1+h_t(x_i,y_i)\,-\,h_t(x_i,y:y\ne y_i))}\\= & {} \left\{ \begin{array}{ll} D_t(i)\alpha _t &{}\text{for } \text{ correctly } \text{ labeled } \text{ examples } \\ D_t(i) &{}\text{for } \text{ misclassified } \text{ examples } \end{array} \right. \end{aligned}$$Higher importance is assigned to the mislabeled examples.
-
7.
Normalize \(D_{t+1}\): \(D_{t+1}(i)=\frac{D_{t+1}(i)}{\sum _i D_{t+1}(i)}\)
Output the final hypothesis:
3 Results and discussion
All data were analyzed using Matlab (MathWorks, Natick, MA).
A cross-validation (CV) technique was used in order to estimate how accurately a predictive model will perform in practice. Figure 1 shows one round of CV which involves partitioning a sample of data into complementary subsets, training and test sets, building the classifier on the first set, and validating the model on the second set. To reduce variability, multiple rounds of CV are performed using different partitions, and the validation results are averaged over the rounds.
Before performing the classification, the preprocessing involved a first registration of all the images in the same stereotaxic space and extraction of the gross peri-hippocampal VOI containing \(50 \times 60 \times 60 = 180000\) voxels (see Sect. 2.1). Next 315 features suitable for describing complex images were extracted, as reported in Sect. 2. Hence the number of examples in the training (test) set was given by 180000 \(\times\) the number of training (test) images, and the number of components was 315. Internally to each round of the cross validation, a bounding box around the training hippocampi was defined by the logical OR of the training masks. A reduced VOI (rVOI) was identified using this bounding box plus some neighboring voxels obtained applying a cubic kernel of size \(2 \times 2 \times 2\). The rVOI dimensions increased with the number m of training images (with \(m = 5, 10, 15,\ldots , 40\)) and in each round of the CV, the rVOIs changed. The rVOI dimensions over ten rounds of CV were averaged. The resulting mean values, varying m, are shown in the Table 3. Reduced training set and test set were built based on the training rVOI; their size can be computed multiplying the rVOI size by the number of training/test images.
The voxels outside the training rVOI definitely do not belong to the hippocampus. The neighboring voxels were included because they might contain hippocampal voxels of testing images lying outside the bounding box. The percentage of hippocampal voxels in the training rVOIs was in the range of 27–38 % of the total number. The use of rVOIs also reduced the computational time required for training the classifiers. It is worth reporting that in a first attempt, random undersampling of the majority class was used to obtain a desired unbalancing (in the range of 25–40 %) between hippocampus and non-hippocampus sets, combined with the classification task. This procedure results in worsened performances of the classifiers, hence the rVOI extraction was adopted.
A number of standard metrics, described in Appendix 1, were calculated for each segmentation algorithm: Dice’s coefficient, Precision, Recall and Relative Overlap (R.O). In particular Dice’s index was used to compare the performances of the methods [12]. Left and right hemispheres were independently analyzed.
The RUSBoost performance for automated segmentation on the DB1 MRI data set was studied. The RUSBoost algorithm provided by the fitensemble function in the Statistics Toolbox of Matlab was used. The relationship between the evaluation metrics and the number m of training VOIs, with \(m=5, 10, 15,\ldots , 40\), was evaluated using the strategy shown in Fig. 1, with 10 CVs. Parameters tuning of RUSBoost was performed on a wide range of values: number of rounds T equal to \(10, 50, 100, 150, 200, 250,\ldots , 500\) and learning rate equal to \(0.01, 0.05, 0.1, 0.2, \ldots , 1.\) The optimal number of rounds was \(T=150\), the learning rate equal to 0.1, and the desired percentage of minority class was set at the default value of \(N=50\,\%\). The results, illustrated in Table 4, highlighted the excellent performances of RUSBoost which provided a Dice’s index of 0.84 with only 10 training images. Its ability to separate hippocampal from background voxels improved as the number of training VOIs increased. The best performances of RUSBoost were obtained with \(m = 30\) training VOIs with a Dice’s coefficient of \(0.88 \pm 0.01\) for the left, and \(0.87 \pm 0.01\) for the right side. The Dice’s coefficient did not improve by increasing further the number of training VOIs, suggesting that \(m = 30\) was the optimal number.
Subsequently, we compared the performances of RUSBoost with two classifiers previously used in medical image analysis [11, 27, 28]: Adaboost and RF (see Appendix 1). Figure 2 summarizes the relationship between the Dice’s coefficients of the three classifiers and the number of training VOIs. Parameters tuning of Adaboost was performed using a number of rounds T equal to \(10, 50, 100, 150, 200, 250,\ldots , 500\) and learning rate equal to \(0.01, 0.05, 0.1, 0.2, \ldots , 1\); the optimal number of boosting rounds was \(T = 400\) and its learning rate 0.1. Parameter tuning of RF was performed using the number of trees equal to \(10, 50, 100, 150, 200, 250,\ldots , 500\) and the optimal number resulted to be 150. The metrics values were estimated performing ten cross validations. The best performance of Adaboost on DB1 data set was reached with few training VOIs, providing a Dice’s index of about 0.77. The figure shows that Adaboost had a limited learning ability, because the Dice’s coefficient did not increase significantly as the number of training examples increased, and its performances were very poor compared with those of RUSBoost and RF. The advantage of combining the RUS with boosting appeared conspicuous. As already seen for RUSBoost, the Dice’s coefficients of the RF classifiers increased with the number of training VOIs and the curves leveled off after 30 training images, indicating that it would be pointless increase further the number of images. The best performances of RF were obtained using \(m = 30\) VOIs with a dice’s index of \(0.87 \pm 0.01\) for left and \(0.86 \pm 0.01\) for right hemispheres, in agreement with RUSBoost results. Table 5 shows all the metrics values obtained using \(m = 30\) training VOIs for left and right hemispheres, highlighting a strong concordance of results between the two brain hemispheres.
RUSBoost showed higher Recall than RF: the \(87\,\%\) (\(86\,\%\)) of true left (right) hippocampus was correctly identified by RUSBoost, versus the \(85\,\%\) (\(82\,\%\)) identified by RF. The Precision with RF was slightly higher than that of RUSBoost: \(89\,\%\) (\(91\,\%\)) of the voxels that RF predicted as hippocampus for the left (right) side, was true hippocampus. This was \(88\,\%\) with RUSBoost.
Finally, in Table 5 the RUSBoost behavior was compared the publicly available segmentation package FreeSurfer v.5.1 (see Appendix 1), highlighting the excellent segmentation performances of the proposed algorithm. FreeSurfer segmentations compared with manual segmentations similarly to Adaboost, with a Dice’s coefficient of 0.74 (0.76) for the left (right) side. These numbers should be treated with caution, because FreeSurfers segmentation tool uses a probabilistic atlas constructed from training data different from those employed for other algorithms, and an exact comparison is not possible without using the same data. To overcome this drawback, the performances of all the segmentation methods were evaluated on an independent data set. With this aim, we used an external data set DB2, obtained from an ADNI archive. This procedure guarantees a bias-free estimations of metrics for the RUSBoost, Adaboost and RF final model, trained on DB1, since DB2 was not employed to select the final models. For this section of the study, FreeSurfer was used again for comparison. The results (Table 6) illustrate the excellent performance of RUSBoost, followed by RF, on DB2, in keeping with the DB1 analysis. Unlike the DB1 analysis, in this case RUSBoots also achieved best Precision (0.89 and 0.88 for left and right side) and Recall (0.86 and 0.85 for left and right side). FreeSurfer and Adaboost gave the worst results.
Figure 3 shows the scatter plots and linear fits of the hippocampal volumes obtained using the manual tracing and the two best automated segmentations measured by RUSBoost and RF. The hippocampal volumes measured by RUSBoost showed the best agreement with the manually segmented volumes with a Pearson correlation coefficient r \(= 0.83\) (0.82) for left (right) side, statistically significant (p value \(= 1 \times 10^{-13}\)). We also performed a paired two-sided sign test of the null hypothesis that the difference between volumes obtained by automated and manual segmentations comes from a continuous distribution with zero median, against the alternative that the distribution does not have zero median. For RF segmentation, the results of the sign test indicated a rejection of the null hypothesis at the \(5\,\%\) significance level, with p value \(= 6.17 \times 10^{-5}\) for the left and p value \(= 3.63 \times 10^{-7}\) for the right side. Hence the hypothesis that the difference between volumes measured by RF segmentation and volumes obtained by manual tracing comes from a continuous distribution with zero median was rejected. For RUSBoost segmentation, at the \(5\,\%\) significant level the test fails to reject the null hypothesis, therefore we cannot reject that the difference between volumes measured by RUSBoost segmentation and volumes obtained by manual tracing comes from a continuous distribution with zero median, with p value \(= 0.152\) for the left and p value \(= 0.253\). Overall, these are very encouraging results for a possible diagnostic use of this method and represent further evidence of the great potential of the proposed strategy for automated tissue segmentation.
Scatter plots of the hippocampal volumes computed by the manual (target) and automated (output) segmentations on left and right brain hemispheres. The automated tracing was performed by RUSBoost and RF algorithms. The linear regressions of target relative to output are plotted and the Pearson regression coefficients (r) between manual and automated volumes are shown
4 Conclusions
The use of automated techniques for image segmentation and analysis is gradually overtaking manual methods, particularly when applied to highly prevalent conditions, such as AD [11] and temporal lobe epilepsy [37], both disorders in which the hippocampus plays a pivotal role in the pathogenesis of the illness.
In this paper, we propose a novel strategy for automated segmentation of the hippocampal region based on the classifier RUSBoost, which produced excellent results when compared with other two learning methods, Adaboost and RF, and the publicly available package, FreeSurfer. For all experiments described in this paper, the classifiers were learning generalizable methods. RUSBoost gave the best results in terms of evaluation metrics; RF was the next best, suggesting that RUSBoost and RF may perform much better than both Adaboost and FreeSurfer.
RUSBoost proved to be the most accurate, with high sensitivity and precision. Moreover, the hippocampal volumes measured by RUSBoost showed the highest, statistically significant correlation with manually segmented volumes.
Some of the differences in the results obtained using different segmentation methods may be ascribed to the fact that the tools have been trained and tuned on different databases. Differences in image quality, manual segmentation protocol, clinical status and demographics have been described as possible causes of discrepancy [38]. An advantage of using machine learning algorithms for segmentation is the opportunity of using very large training data sets, shared by the scientific community. This is exemplified by the efforts of the EADC-ADNI working group to develop a standard harmonized protocol for the manual segmentation [8, 31, 32] (http://www.hippocampal-protocol.net) employed in our analysis.
This study was performed blindly to subject status. In terms of further developments, future efforts will be devoted to the application of these techniques to multiple data sets and other illness models. This approach could be extended to the study of other anatomical structures that have proved rather elusive to accurate segmentation, such as the thalamus or the putamen, both complex deep gray matter structures.
Overall, the results obtained with automated segmentation are very promising and a better understanding of the characteristics of the main machine learning methods is necessary for future applications combining multiple biomarkers and different illness sub-types.
References
International A.D (2013) World Alzheimer Report 2013 Overcoming the stigma of dementia
Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of alzheimer’s disease: revising the nincdsadrda criteria. Lancet Neurol 6:734–746
Bruno S, Cercignani M, Wheeler-Kingshott C (2012) Neurodegenerative dementias: from MR physics lab to assessment room. Eur Phys J Plus 127:1–15
Bellotti R, Pascazio S (2012) Editorial: advanced physical methods in brain research. European Physical Journal Plus 127:1–2
Weiner M, Veitch D, Aisen P, Beckett L, Cairns N, Green R, Harvey D, Jack C, Jagust W, Liu E, Morris J, Petersen R, Saykin A, Schmidt M, Shaw L, Siuciak J, Soares H, Toga A, Trojanowski J (2012) The Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dementia 8:61–68
Prestia A, Boccardi M, Galluzzi S, Cavedo E, Adorni A, Soricelli A, Bonetti M, Geroldi C, Giannakopoulos P, Thompson P, Frisoni G (2011) Hippocampal and amygdalar volume changes in elderly patients with alzheimer’s disease and schizophrenia. Psychiatry Res 192(2):77–83
Chincarini A, Bosco P, Gemme G, Morbelli S, Arnaldi D, Sensi F, Solano I, Amoroso N, Tangaro S, Longo R, Squarcia S, Nobili F (2012) Alzheimer’s disease markers from structural MRI and FDG-PET brain images. Eur Phys J Plus 127:1–16
Frisoni G, Jack C (2011) Harmonization of magnetic resonance-based manual hippocampal segmentation: a mandatory step for wide clinical use. Alzheimers Dement 7(2):171–4
Wang H, Suh JW, Das SR, Pluta J, Craige C, Yushkevich PA (2013) Multi-atlas segmentation with joint label fusion. Anal Mach Intell 35:611–623
Cootes T, Taylor C, Cooper D, Graham J (1995) Active shape models-their training and applications. Comput Vis Image Underst 61:38–59
Morra J, Tu Z, Apostolova L, Green A, Toga A, Thompson P (2010) Comparison of adaboost and support vector machines for detecting alzheimer’s disease through automated hippocampal segmentation. IEEE Trans Med Imaging 29:30–43
Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR Jr, Weiner MW, Thompson PM (2008) Validation of a fully automated 3d hippocampal segmentation method using subjects with alzheimer’s disease mild cognitive impairment, and elderly controls. Neuroimage 43(1):59–68
Balafar M, Ramli A, Saripan M, Mashohor S (2010) Review of brain MRI image segmentation methods. Artif Intell Rev 33:261–274
Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HW II, Lewis DV, LaBar KS, Styner M, McCarthy G (2009) A comparison of automated segmentation and manual tarcing for quantifying hippocampal and amygala volumes. Neuroimage 45(3):855–866
Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale A (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neurotechnique 33:341–355
Bendib M, Merouani H, Diaba F (2014) Automatic segmentation of brain mri through stationary wavelet transform and random forests. Pattern Anal Appl doi:10.1007/s10044-014-0373-y
Patenaude B, Smith S, Kennedy D, Jenkinson M (2011) A bayesian model of shape and appearance for subcortical brain. Neuroimage 56(3):907–922
Ortiz A, Gorriz J, Ramirez J, Salas-Gonzalez D, the Alzheimer’s Disease Neuroimaging Initiative F (2012) Improving mri segmentation with probabilistic ghsom and multiobjective optimization. Neurocomputing 114:118–131
Bron E, Smits M, van der Flier WM et al (2015) Standardized evaluation of methods for computer-aided diagnosis of dementia based on structural MRI: the CSDDementia challenge. Neuroimage (in press)
Seiffert C, Khoshgoftaar T, Hulse J, Napolitano A (2010) Rusboost: a hybrid approach to alleviating class imbalance. IEEE Trans Syst Man Cybern 40:185–197
Chawla N, Lazarevic A, Hall L, Bowyer K (2003) Smoteboost: improving prediction of the minority class in boosting. In: 7th European conference on principles and practice of knowledge discovery in database pp 107–119
Talln-Ballesterosa A, Hervs-Martfnezb C, Riquelmea J, Ruiz R (2013) Feature selection to enhance a two-stage evolutionary algorithm in product unit neural networks for complex classification problems. Neurocomputing 114:107–117
Cui Y, Ma H, Saha T (2014) Improvement of power transformer insulation diagnosis using oil characteristics data preprocessed by smoteboost technique. IEEE Trans Dielectr Electr Insul 21:2363–2373
Govindaraj M, Lavanya S (2013) A combined boosting and sampling approach for imbalanced data classification. Int J Adv Res Data Min Cloud Comput 1:44–50
Freund Y, Schapire R (1997) A decision-theoretic generalization of on-line learning and an application to boosting. J Comput Syst Sci 55:119–139
Breiman L (2001) Random forest. Mach Learn 45:5–32
Tangaro S, Amoroso N, Boccardi M, Bruno S, Chincarini A, Ferraro G, Frisoni G, Maglietta R, Redolfi A, Rei L, Tateo A, Bellotti R (2014) Automated voxel-by-voxel tissue classification for hippocampal segmentation: methods and validation. Phys Med 30:878–887
Maglietta R, Amoroso N, Bruno, S., Chincarini, A., Frisoni, G., Inglese, P., Tangaro, S., Tateo, A., Bellotti, R.: Random forest classification for hippocampal segmentation in 3d mr images. In: 12th international conference on machine learning and applications (2013) 264–267
Chyzhyk D, Dacosta-Aguayo R, Mataro M, Grana M (2015) An active learning approach for stroke lesion segmentation on multimodal mri data. Neurocomputing 150:26–36
Sabuncu MR, Yeo BT, Van Leemput K, Fischl B, Golland P (2010) A generative model for image segmentation based on label fusion. IEEE Trans Med Imaging 29(10):1714–1729
Boccardi M, Bocchetta M, Apostolova L, Barnes J, Bartzokis G, Corbetta G, DeCarli C, DeToledo-Morrell L, Firbank M, Ganzola R, Gerritsen L, Henneman W, Killiany R, Malykhin N, Pasqualetti P, Pruessner J, Redolfi A, Robitaille N, Soininen H, Tolomeo D, Wang L, Watson H, Wolf H, Duvernoy H, Duchesne S, Jack C, Frisoni G, for the EADC-ADNI Working Group on the Harmonized Protocol for Manual Hippocampal Segmentation (2015) Delphi definition of the eadc-adni harmonized protocol for hippocampal segmentation on magnetic resonance. Alzheimer’s and Dementia 11:126–138
Frisoni GB, Jack C, Bocchetta M, Bauere C, Frederiksenf K, Liug Y et al (2015) The eadc-adni harmonized protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimer’s Dementia 11:111–125
Viola P, Jones M (2001) Rapid object detection using a boosted cascade of simple features. In: Computer vision and pattern recognition, 2001. CVPR 2001. Proceedings of the 2001 IEEE computer society conference on. vol 1, IEEE pp I–511
Haralick R, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3(6):610–621
Tesar L, Shimizu A, Smutek D, Kobatake H, Nawano S (2008) Medical image analysis of 3 D CT images based on extension of haralick texture features. Comput Med Imaging Graph 32:513–520
Tangaro S, Amoroso N, Brescia M, Cavuoti S, Chincarini A, Errico R, Inglese P, Longo G, Maglietta R, Tateo A, Riccio G, Bellotti R (2015) Feature selection based on machine learning in mris for hippocampal segmentation. Comput Math Methods Med 2015:10. doi:10.1155/2015/814104
Focke N, Yogarajah M, Symms M, Gruber O, Paulus W, Duncan J (2012) Automated MR image classification in temporal lobe epilepsy. Neuroimage 59(1):356–362
Lotjonen JMP, Wolz R, Koikkalainen JR, Thurfjell L, Waldemar G, Soininen H, Rueckert D, The Alzheimer's Disease Neuroimaging Initiative (2010) Fast and robust multi-atlas segmentation of brain magnetic resonance images. Neuroimage 49(3):2352–2365
Dale A, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Med Imaging 20:70–80
Fischl B, Salat DH, van der Kouwe AJ, Makris N, STgonne F, Quinn BT, Dale AM (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23:S69–S84 (Mathematics in brain imaging)
Fischl B, Sereno MI, Dale A (1999) Cortical surface-based analysis: Ii: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207
Fischl B, Sereno MI, Tootell RB, Dale AM (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284
Fischl B, van der Kouwe A, Destrieux C, Halgren E, STgonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cerebral Cortex 14:11–22
Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B (2006) Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32:180–194
Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J, Fischl B, Dale A (2006) Reliability in multi-site structural mri studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30:436–443
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in mri. Neuroimage 22:1060–1075
Dale A, Sereno M (1993) Improved localization of cortical activity by combining eeg and meg with mri cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176
Reuter M, Rosas HD, Fischl B (2010) Highly accurate inverse consistent registration: a robust approach. Neuroimage 53:1181–1196
Sled J, Zijdenbos A, Evans A (1998) A nonparametric method for automatic correction of intensity nonuniformity in mri data. IEEE Trans Med Imaging 17:87–97
Segonne F, Pacheco J, Fischl B (2007) Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 26:518–529
Desikan RS, STgonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on mri scans into gyral based regions of interest. Neuroimage 31:968–980
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701
Kuperberg GR, Broome M, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams S, van der Kouwe A, Salat D, Dale A, Fischl B (2003) Regionally localized thinning of the cerebral cortex in Schizophrenia. Archives of General Psychiatry 60:878–888
Salat D, Buckner R, Snyder A, Greve DN, Desikan R, Busa E, Morris J, Dale A, Fischl B (2004) Thinning of the cerebral cortex in aging. Cerebral Cortex 14:721–730
Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012) Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61:1402–1418
Reuter M, Fischl B (2011) Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 57:19–21
Acknowledgments
Data used in preparation of this article were obtained from the Alzheimers Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California, San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the USA and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO and ADNI-2. To date these three protocols have recruited over 1500 adults, ages 55 to 90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow-up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see http://www.adni-info.org. Data collection and sharing for this project were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimers Association; Alzheimers Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. We are grateful to A. Argentieri and R. Colella for technical assistance and P. Soria for graphical work.
Conflict of interest
All authors disclose any actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations that could inappropriately influence their work. All experiments were performed with the informed consent of each participant or caregiver in line with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Local institutional ethics committees approved the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
For The Alzheimers Disease Neuroimaging Initiative refer Acknowledgments
Appendices
Evaluation metrics
A number of standard metrics described below were used to compare the performances of the four segmentation algorithms. Two binary vectors A and B are considered. A contains the voxel labels as identified by manual tracing and B contains the voxel labels predicted using a supervised learning algorithm. The voxels that the classifier correctly identifies as belonging to the hippocampus represent the true positives (TP) (i.e. intersection of A and B), the voxels correctly identified as background the true negatives (TN); the voxels wrongly identified as belonging to the hippocampus are the false positives (FP), and, finally, the voxels wrongly identified as background are the false negatives (FN).
Dice’s coefficient, precision, recall and relative overlap are defined as follows:
The Dice’s coefficient is an agreement measure over two sets of measures, A and B, defined as two times the ratio of the intersection of the two sets (i.e. TP) on the sum of A and B. Precision is defined by the ratio of the number of correct positive predictions on the number of total positive predictions. Recall measures the proportion of actual positives correctly identified from the number of all the actual positive examples. Relative overlap (R.O.) measures the similarity between two sets of measures as the size of the intersection divided by the size of the union of the sets.
Classifiers
1.1 Adaboost
Adaboost is a meta-algorithm that sequentially selects weak classifiers, and weighs each of them based on their error. A weak classifier is a classifier that performs better than pure chance. The algorithm assigns to each example the weight \(D_1(i) = \frac{1}{m}\). Then, in each round \(t = 1, 2, \ldots , T\), the following steps are performed:
-
1.
Training of a weak learner \(h_t: X \rightarrow \{-1, +1\}\) using the distribution \(D_t\).
-
2.
Calculation of the error \(e_t = \sum _{i:h_t( x_ i)\ne y_i} D_t(i)\).
-
3.
Setting of \(\alpha _t = \frac{1}{2}\text {ln}\left(\frac{1-e_{\it {t}}}{e_{\it {t}}}\right)\), which measures the importance assigned to \(h_t\). If \(e_t \le \frac{1}{2}\) then \(\alpha _t \ge 0\).
-
4.
Setting of \(D_{t+1}(i) = \frac{D_t(i)}{Z_t}e^{-\alpha _t y_i h_t({\mathbf x} _i)}\), where \(Z_t = 2\sqrt{e_t(1-e_t)}\) is a normalization factor. \(D_t(i)\) measures the importance assigned to the example \(x_i\) at the iteration t.
The output of the strong classifier on a new example \(\mathbf x\) is:
The algorithm tends to concentrate on hard examples, i.e. after selecting an optimal classifier \(h_{t}\) for the distribution \(D_{t}\), the examples \(x_{i}\), that were identified correctly by the classifier \(h_{t}\), are given lower weight, and those that were identified incorrectly by \(h_{t}\) are given higher weight. Therefore, when the algorithm is testing the classifiers on the distribution \(D_{t+1}\), it will select a classifier that better identifies those examples that the previous classifier missed.
The final hypothesis y is a weighted majority vote of the T weak hypothesis where \(\alpha _t\) is the weight to \(h_t\), that is the weighted mean of the T weak classification on \(\mathbf x\).
1.2 Random forest
Random Forest uses multiple binary decision trees. Each of the classification trees is built using a sample of the training data, and at each node a randomly chosen set of variables is considered for the best split.
For \(b = 1, 2, \ldots, B\) the RF algorithms can be briefly described as follows.
-
1.
A bootstrap sample Z* of size n is drawn from the training set.
-
2.
A random forest tree \(T_b\) is grown from the bootstrapped data, by recursively repeating the following steps for each terminal node of the tree, until the minimum node size, \(n_{min}\), is reached:
-
Selection of q variables at random from the d variables;
-
Choice of the best variable/split-point among q (internal feature selection);
-
Splitting of the node into two daughter nodes.
-
-
3.
Output of the ensemble of trees \(\{T_b\}_1^B\).
Given a new point \(\mathbf x\), let \(\tilde{C}_b(\mathbf x )\) be the class prediction of the \(b-\)th random forest tree, the prediction of RF on this new sample is given by
In the experiments here described, \(q = \sqrt{d}\) and the minimum node size was 1.
FreeSurfer
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite, which is documented and freely available for download online.Footnote 1 The technical details of these procedures are described in prior publications [15, 39–49]. Briefly, this processing includes motion correction and averaging [50] of multiple volumetric T1 weighted images (when more than one is available), removal of non-brain tissue using a hybrid watershed/surface deformation procedure [48], automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) [15, 42] intensity normalization [51], tessellation of the gray matter white matter boundary, automated topology correction [41, 52], and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [39, 40, 49]. Once the cortical models are complete, a number of deformable procedures can be performed for in further data processing and analysis including surface inflation [39], registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across subjects [44], parcellation of the cerebral cortex into units based on gyral and sulcal structure [45, 53], and creation of a variety of surface-based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface [40]. The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data thus are capable of detecting submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis [54] and manual measurements [55, 56]. Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths [46, 57].
1.1 Example text for longitudinal processing
To extract reliable volume and thickness estimates, images where automatically processed with the longitudinal stream in FreeSurfer [57]. Specifically an unbiased within-subject template space and image [58] is created using robust, inverse consistent registration [50]. Several processing steps, such as skull stripping, Talairach transforms, atlas registration as well as spherical surface maps and parcellations are then initialized with common information from the within-subject template, significantly increasing reliability and statistical power [57].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maglietta, R., Amoroso, N., Boccardi, M. et al. Automated hippocampal segmentation in 3D MRI using random undersampling with boosting algorithm. Pattern Anal Applic 19, 579–591 (2016). https://doi.org/10.1007/s10044-015-0492-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10044-015-0492-0