Abstract
Purpose
Cytoreductive surgery (CRS) is often combined with hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of peritoneal tumour deposits. Considering CRS, the evidence relating the large incisions, local chemotherapy and abdominal wall trauma to incisional hernias (IH) has not been synthesized. This systematic review and meta-analysis was conducted to examine the proportion of IH present in patients post CRS and the effect HIPEC had on these rates.
Methods
PubMed, EMBASE, and Cochrane Central Registry of Trials were searched up to June 2023 to examine studies relating IH and CRS plus or minus HIPEC. The most up to date PRISMA guidelines were followed. Pertinent clinical information was synthesized in tabular form. A meta-analysis reporting the pooled proportions of IH post CRS plus or minus HIPEC, the odds of IH in HIPEC versus non-HIPEC CRS and the difference in follow-up time between groups was conducted.
Results
Nine studies comprising 1416 patients were included. The pooled proportion of IH post CRS was 12% (95% confidence interval (CI) 8–16%) in HIPEC and 7% (95% CI 4–10%) in non-HIPEC patients and 11% (95% CI 7–14%) overall. Previously reported rates of IH in midline laparotomy range from 10 to 30%. The odds of IH in the HIPEC was 1.9 times higher compared to non-HIPEC cohorts however this was not statistically significant (odds ratio (OR) 1.9, 95% 0.7–5.2; p = 0.21). There was no significant difference in average follow-up times between HIPEC and non-HIPEC cohorts.
Conclusions
IH post CRS plus or minus HIPEC were in the expected range for midline laparotomies. IH in patients receiving HIPEC may occur at a greater proportion than in non-HIPEC patients, however, there were too few studies in our meta-analysis to determine this with statistical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic/heated intraperitoneal chemotherapy (HIPEC) is an effective management strategy for advanced peritoneal malignancies [1,2,3]. CRS aims for complete tumour removal, involving extensive peritoneal and visceral resection [4]. Once optimal cytoreduction has been achieved, HIPEC is employed intraoperatively and, in select cases, is followed by early postoperative intraperitoneal chemotherapy (EPIC) [5, 6]. However, it is associated with complications including bowel perforation, anastomotic leak and incisional hernias (IH), alongside a postoperative morbidity and mortality reported in the range 22–41% and 2–5%, respectively [7,8,9,10,11,12]. The overall incidence of IH in those undergoing laparotomy has been documented in the literature to exceed 20% [13,14,15,16]. Late morbidity and in particular the occurrence of an IH have not been well studied in those with peritoneal malignancies managed with CRS/HIPEC [17, 18].
Although the true incidence is unclear, several studies have reported an IH incidence between 7 and 17% [19,20,21]. CRS/HIPEC represents a complex surgical intervention of considerable duration [22]. Notwithstanding, this procedure poses a potential risk for hernia development, given several inherent factors. Primarily, a significant proportion of CRS/HIPEC patients have a history of previous abdominal surgeries, a factor well-documented to increase hernia susceptibility due to abdominal wall weakening [23, 24]. Moreover, the lengthy duration of the CRS/HIPEC procedure necessitates sizable incisions, thereby subjecting the abdominal wall to heightened stress and augmenting the likelihood of herniation [22, 25, 26]. The intraperitoneal delivery of chemotherapy during CRS/HIPEC can result in immunosuppression, further compromising abdominal wall integrity [21, 27]. Nonetheless, the precise proportion of patients developing an IH following CRS/HIPEC remains largely unexplored, as existing studies predominantly focus on short-term morbidity and long-term oncological outcomes [28].
Understanding the proportion of IH, risk factors, and outcomes related to IH post-CRS/HIPEC is essential for risk assessment, prevention, and optimal management. Further research is needed to refine preventitive strategies, standardize surgical techniques, and assess long-term outcomes to enhance patient care. The aims and learning points of this systematic review and meta-analysis is to assess the proportion of patients, risk factors and outcomes in patients who develop IH post CRS with or without HIPEC and how this information can be utilized to enhance clinical decision making for the betterment of patient outcomes and quality of life.
Methods
Registration and search strategy
Our search was conducted in line with the most recent preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations [29]. Our study protocol was prospectively registered with PROSPERO under the following registration number: CRD42023432188. We conducted a search using PubMed, EMBASE and Cochrane Central Register of Controlled Trials using the search algorithms provided below on the 5th June 2023.
(Peritonectomy OR CRS OR cytoreductive surgery) AND (incision* AND hernia*)
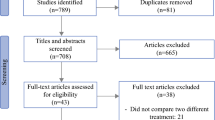
The complete breakdown of analyzed studies can be viewed in the PRISMA diagram in Fig. 1. The bibliographies of included publications were also searched for any relevant studies.
Inclusion criteria:
-
Patients aged 18 years old and above.
-
Underwent CRS/Peritonectomy for oncological purposes plus or minus HIPEC.
-
Prospective or Retrospective Studies.
-
English language or translation available.
-
Use of closure with or without a mesh support device, both primary closure and component separation techniques were acceptable.
-
Reoperation cases due to tumour recurrence.
-
Follow-up post CRS greater than, or equal to 12 months, on average.
Exclusion criteria:
-
Laparoscopic cases.
-
Case series/reports.
-
Consensus statements.
-
Non-IH.
-
Conference abstracts.
-
Non-abdominal wall related surgical procedures e.g., posterior pelvic wall CRS.
-
Early reoperations as a result of initial surgery complications.
-
Missing/conflicting data with no response from contacted authors.
Identification of studies and outcomes of interest
The following population, intervention, comparison, outcome (PICO) elements were used as the basis for selecting studies [30]:
-
Population: Patients undergoing CRS.
-
Intervention: CRS or peritonectomy plus or minus HIPEC.
-
Comparison: Patients whom also underwent CRS plus or minus HIPEC.
-
Outcome: Development of IH post operation.
Studies were independently reviewed by three separate authors (BMC, WQ, HT) using Rayyan [31]. If there was any disagreement between authors, a fourth author (ZQN) was used to mediate the discussion and consensus was reached.
Our primary outcome of interest were the development of IH post CRS plus or minus HIPEC.
Secondary outcomes were risk factors and patient outcomes in relation to the development of IH post CRS plus or minus HIPEC.
Data extraction
Relevant metrics and information were extracted using a template on Google Sheets (Mountain View, California, United States). Three independent authors (WQ, BMC, HT) were involved in the data extraction.
Study selection
No randomized trials have been completed on the topic to the best of the author’s knowledge. Retrospective or prospective observational studies examining IH post CRS plus or minus HIPEC with at least 12 months follow-up time on average, were of interest. Where differing closure types within the same study without mesh were utilized these results were pooled into the same analysis. Only one included study reported mesh use and as such this mesh cohort was excluded from the meta-analysis [32]. One study included a small cohort (5% of patients) whom received “intraperitoneal chemotherapy” with 95% of patients not receiving any. These patients were not differentiated in terms of IH outcomes and for the purpose of this analysis all patients were classed as non-HIPEC [33]. Only first time CRS/HIPEC patients in the study authored by Wong et al. were included in our analysis due to missing data and heterogeneity of results for their repeat cohort [34].
Risk of bias assessment
Assessment of potential biases for the non-randomised studies was assessed using a modified Newcastle–Ottawa scale risk of bias tool [35], with the results tabulated as in Table 1. This assessment tool grades each study as being ‘satisfactory’ or ‘unsatisfactory’ across various categories. We assigned stars to evaluate study quality: 7–8 stars—“very good”, 5–6 stars “good”, 3–4 stars “satisfactory” and 0–2 stars “unsatisfactory”. The critical appraisal was completed by two reviewers independently (BMC and HT), where once again a third reviewer (WQ) was asked to arbitrate in cases of discrepancies in opinion.
Statistical analysis
We performed a proportional meta-analysis as part of this review [36]. Statistical analysis was run using Stata 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). The proportion of patients developing IH post CRS plus or minus HIPEC was pooled using the “metaprop” function within Stata [37]. 95% confidence intervals (CI) were employed and p ≤ 0.05 was considered statistically significant. Heterogeny was reported using I2 [37]. We considered there to be a notable degree of heterogeny if I2 was greater than 50% [38]. A random effects model was used due to evidence of significant statistical heterogeneity as well evidence of study design heterogeneity [39].
To assess publication bias, funnel plots were generated. These are not included in this article as recommended in the literature, due to less than 10 papers being included in the analysis, thus making it an inaccurate representation of publication bias [40]. Qualitative bias assessment was also conducted as proposed by Barker et al. as this is a proportional meta-analysis [36]. If missing data or conflicting data were found upon review of included papers authors were contacted for clarification.
The relationship between HIPEC and non-HIPEC IH proportions was examined using the “metafor” package in R v4.1 [41]. (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.URL https://www.R-project.org/), as previously described [42]. To assess whether follow-up time could be responsible for differences in IH, an independent student’s t test was used to examine the mean follow-up times in relation to non-HIPEC and HIPEC groups. Where studies reported a median and range the mean was estimated using the method put forward by Wan et al. [43]. If follow-up was reported at a set time point, for example one year, this was taken as the mean. If a study reported a minimum follow-up period, this was also taken as the mean for the purpose of follow-up analysis.
Results
Our search yielded ninety four articles of which nine studies were selected for data extraction [10, 19,20,21, 32,33,34, 44]. Studies selected were published between 2014 and 2023, conducted in six countries. A total of 1416 patients were included in our analysis. Study characteristics and patient demographics are found in Table 2 and 3, respectively. All but one study was conducted retrospectively [19]. All but one study took place at a single institution [33]. One study did not specify its location but collected data from a prospectively maintained database [32]. Eight studies included patients who underwent both CRS and HIPEC. Spencer et al. included patients who underwent CRS only [33]. Cascales Campos et al. described two groups, CRS only and a group who underwent both CRS and HIPEC [10]. Patients who underwent HIPEC received variable regimens, but all with either platinum agents such as oxaliplatin/cisplatin, mitomycin or both. Pathologies were wide ranging with the majority described as ovarian cancer, peritoneal mesothelioma, colorectal cancer and appendiceal cancer. Three studies included recurrent disease [10, 20, 32]. Spencer et al. and Wong et al. reported on ovarian cancer and mesothelioma in isolation, respectively [33, 34].
Incisional hernia
Overall, 148 incisional hernias occurred within the included studies. Six studies diagnosed post operative IH through clinical and radiological assessment, whilst Wenzelberg et al. used CT imaging solely for diagnosis [27]. Wong et al. and Parikh et al. did not specify the diagnostic method [32, 34].
In the pooled proportion of CRS/HIPEC patients, IH occurred in 12% (95% CI 8–16%). Significant heterogeneity was found between studies (I^2 75.24%, p < 0.01).
Risk factors
A wide range of risk factors were identified in their contribution to IH formation. Patient pathology was identified as a significant risk factor by Struller et al. with pseudomyxoma peritonei and peritoneal mesothelioma patients at higher risk of developing IH (OR 4.295 p = 0.022) [20]. Three studies found patient characteristics such as old age, female sex and increased BMI > 30 were significant risk factors [19, 21, 33]. Two studies examined closure techniques post CRS/HIPEC, and found an increased 4:1 suture to wound length ratio was beneficial for prevention of IH (p = 0.048), whilst the use of mesh was not effective [32, 44]. Wenzelberg described cardiovascular disease as a significant risk factor for IH formation (p = 0.024) [27]. Spencer et al. identified poor pre-op nutritional status as a risk factor for IH occurrence in the first year of follow-up (p < 0.001), whilst Cascales Campos et al. identified pre-op chemotherapy as a risk factor (p = 0.041) [10, 33]. Wong et al. did not describe risk factors for IH formation [34].
Non IH reported patient outcomes
The studies included reported heterogenous outcomes. No study identified CRS with HIPEC as independent risk factors for IH formation on multivariate analysis. Patients with IH had significantly decreased quality of life compared to those who did not develop IH using the Short Form Survey-36 tool in the domains of Role-physical and Role-emotional [19]. Parikh et al. identified wound complications such as dehiscence and wound infection as significant comorbidities in patients requiring abdominal wall resection during CRS/HIPEC (p = 0.0032) [32]. No studies reported on overall survival outcomes relating to IH. Further information pertaining to chemotherapy regimen used is reported in Table 4.
Meta-analysis
Pooled proportions of IH
Nine studies were included in the pooled analysis. The pooled proportion of patients whom developed an IH post CRS plus or minus HIPEC. The pooled proportion of patients developing an IH in the cohort receiving HIPEC was 12% (95% confidence interval CI 8–16%). The pooled proportion of patients developing an IH in the cohort non receiving HIPEC was 7% (95% CI 4–10%). There was significant heterogeneity between studies with an I2 = 78.32% (p < 0.01). Overall, the proportion of CRS plus or minus HIPEC patients developing an IH was 11% (95% CI 7–14%). The results are visually described in Fig. 2. Of note, studies subjected patients to differing follow-up times as described in Table 3.
Odds of IH in HIPEC and Non-HIPEC cohorts
We report an odds ratio (OR) and 95% CI relating the odds of developing an IH in patients whom underwent HIPEC compared to patients who did not. In the pooled HIPEC cohort, patients had nearly twice the odds of IH (OR = 1.9, 95% CI 0.7, 5.2) when compared to non-HIPEC cohorts. However, there is no strong evidence for this effect at a generalisable population level, since p = 0.21 and the CI includes 1 (a null ratio). Our interval is quite wide, with 30% lower odds of IH in HIPEC or up to 5.2 times higher odds of IH in HIPEC possible.
Difference in follow-up times
An independent samples t test was used to examine the relationship between follow-up times in the HIPEC and non-HIPEC cohorts, as this may skew results. Results are as observed in Fig. 3.
We can see a mean follow-up of 18 months in the non-HIPEC group and 30.1 months in the HIPEC group. This results are not statistically significant, p = 0.53. This is visually illustrated in Fig. 4. Here, we can observe the CI of the two groups overlapping, and the median value, below that of the mean in the HIPEC group, possibly indicating skewed data.
Discussion
We performed a systematic review and meta-analysis regarding the occurrence of IH post CRS plus or minus HIPEC. From our results, we report a pooled proportion of patients developing an IH of 12% in the HIPEC group, 7% in the non-HIPEC group and 11% overall. There was evidence of statistical heterogeneity in the HIPEC group and between groups. Considering the odds of developing an IH post CRS/HIPEC we reported an OR of 1.9, which was not statistically significant, indicating further research is required to determine clinical significance. These results indicate that IH may be more likely in the HIPEC group. We also observe no statistically significant difference between mean follow-up times in HIPEC or non-HIPEC groups, which can affect the rates of IH observed [45].
Rates of IH post midline laparotomy, not specifically related to CRS, of 10–30% have been described [46]. The pooled proportion of IH post CRS plus or minus HIPEC of 11% is at the lower end of expected rates. This may be due to a number of reasons, including closure technique, BMI, previous surgery, age and gender [47,48,49,50]. As well as this, the actual rates of IH post CRS plus or minus HIPEC may be higher. Considering midline laparotomy in general, only 75% of IH were seen to occur within 2 years of surgery in previous studies [48].
Beadles et al. have shown incidence rates of IH emergency repair in elderly women and men of 23.5 and 32.0 per 100,000 population in the United States, respectively [51]. This serves to highlight the impact IH can have on patient outcomes, and healthcare systems.
In an obese cohort undergoing midline laparotomy, required IH repairs rates of 29% have been reported in the literature [52], with the rate of incarcerated IH repair reported as 3.7% [53]. The expected rates of IH in midline laparotomy in conjunction with peritonectomy may be expected to be higher. Within the included studies, 11 out of 28 patients underwent surgical correction of their IH, with one surgery classed as an emergency due to incarceration [10]. Tuttle et al. reported 10 patients whom underwent surgical repair out of 26 IH [21]. 4 out of 14 IH were repaired in Ravn et al.’s publication, with one case classed as an emergency obstruction [19]. 12 out of 19 IH were repaired electively, in Struller et al.’s study [20]. 7 from 265 patients underwent non-emergency IH repair in an ovarian cancer cohort [33]. What must be considered is the benefit of CRS and HIPEC in contrast with the risks of emergency IH repair and morbidity associated with this procedure, in an immunosuppressed patient population.
Regarding ventral hernias, laparoscopic as opposed to open cases have been described as a more cost effective method of repair when hernias recur, however, all are economically costly [54]. In the case of IH repair post peritonectomy, open surgery may be the most effective option due to the fact it may no longer be possible to place a pre-peritoneal mesh. Additionally a retro-rectus approach may not be feasible if the posterior rectus sheath is resected, leaving the option of an onlay repair, which has its own complications [55, 56]. If open repair is undertaken this will further increase repair economic cost.
Our review also identified risk factors that may suggest patients are more likely to develop an IH as described in Table 3.
The primary malignancy was seen to affect IH rates post CRS/HIPEC, with pseudomyxoma peritonei and mesothelioma patients more likely to develop an IH (p = 0.022) [20]. A colorectal primary has also been described as a risk factor for IH by Cascales Campos et al. (p = 0.01), while Spencer et al. details a suboptimal CRS as a risk factor (p < 0.001), which may be considered a surrogate of primary cancer aggressiveness [10, 33]. Nutritional status was also reported as a risk factor for IH in one study (p < 0.001) [10], which is in agreement with previous literature regarding inguinal hernias [57]. Peritoneal cancer/carcinomatosis index (PCI) has been described as accurate in predicting outcomes, however, others have questioned its benefit [58, 59]. Parikh described a PCI greater than 20 as a high burden of disease, but failed to show statistical significance in relation to wound complications post CRS, however, they did not specifically analyze PCI in relation to IH [32]. Wong et al. also reported the effect of PCI on outcomes. They did not analyze PCI in relation to IH but did find PCI > 20 to correlate with overall survival [34]. Of note, our included studies did not report the effect of stoma formation on IH rates, however, previous research has shown rates of anastomotic leak and prognosis seem to be within the established range when stomas are fashioned in CRS [60, 61]. Further research relating stoma formation to IH outcomes may be clinically useful.
The use of meshes in patients with peritoneal metastases has been questioned [62]. However, the use of mesh reconstruction in patients post CRS/HIPEC/laparotomy has been shown to be safe and effective [46, 63, 64]. Only a small cohort of patients in one study included in this analysis reported mesh use [32], however, ongoing studies (ClinicalTrials.gov identifier: NCT03953365) relating to the outcomes regarding mesh use post CRS/HIPEC may further enhance patient outcomes regarding IH. One study included in our analysis did not show a IH development rate that was statistically significant between mesh and no mesh groups [32].
The major limitation of this meta-analysis is an inherent limitation of each of the included studies. The follow-up time was likely insufficient to detect all IH post surgeries. The HIPEC group had a mean follow-up of 30 months and the non-HIPEC group had follow-up of 18 months, falling short of the recommended minimum follow-up period of 36 months [45]. Another limitation is the lack of standardisation in follow-up times, and while we utilized 12 months as an inclusion minimum there is likely to be a difference in IH picked up with longer follow-up, however, in this patient cohort longer follow-up may be difficult due to patient mortality prior to IH development [19,20,21]. Previously described limitations of the statistical methods are also valid [39]. Due to the low volume of papers describing non-HIPEC cohorts this meta-analysis is likely underpowered to detect all outcome differences between HIPEC and non-HIPEC groups, and there is a risk of type II error occurring as a result. Further studies may consider evaluating the clinical significance of HIPEC versus non-HIPEC IH rates.
The proportion of patients developing an IH post CRS plus or minus HIPEC is in the range expected, considering midline laparotomies in general. This analysis suggested that HIPEC may contribute to a greater proportion of patients developing an IH, however, this finding was not statistically significant. Further studies may be clinically useful to further investigate HIPEC’s role in IH development.
References
Sugarbaker PH (2006) New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 7(1):69–76
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN et al (2012) Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30(20):2449–2456
Ray MD, Dhall K (2021) Hyperthermic intraperitoneal chemotherapy (HIPEC) in the management of peritoneal surface malignancies—An evidence-based review. Curr Probl Cancer 45(6):100737. https://doi.org/10.1016/j.currproblcancer.2021.100737. (PMID: 34116836)
Mehta SS, Bhatt A, Glehen O (2016) Cytoreductive surgery and peritonectomy procedures. Indian J Surg Oncol 7(2):139–51. https://doi.org/10.1007/s13193-016-0505-5. (PMID: 27065704; PMCID: PMC4818624)
Sparks DS, Morris B, Xu W, Fulton J, Atkinson V, Meade B, Lutton N (2015) Cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis secondary to mucinous adenocarcinoma of the appendix. Int Surg 100(1):21–28. https://doi.org/10.9738/INTSURG-D-14-00089.1. (PMID:25594636;PMCID:PMC4301290)
McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ (2013) HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol 107(6):591–596
Yan TD, Black D, Savady R, Sugarbaker PH (2007) A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 14(2):484–492
Kusamura S, Baratti D, Virzì S, Bonomi S, Iusco DR, Grassi A et al (2013) Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol 107(4):312–319
Mehta SS, Gelli M, Agarwal D, Goéré D (2016) Complications of cytoreductive surgery and HIPEC in the treatment of peritoneal metastases. Indian J Surg Oncol 7(2):225–229
Cascales Campos PA, González-Gil A, Gómez-Ruiz AJ, Gil-Gómez E, Alconchel-Gago F, Navarro-Barrios A et al (2020) Risk factors and management of incisional hernia after cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal surface malignancies. Hernia 24(2):257–263
Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD et al (2008) Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 15(3):754–763
Baratti D, Kusamura S, Laterza B, Balestra MR, Deraco M (2010) Early and long-term postoperative management following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Gastrointest Oncol 2(1):36–43
Kingsnorth A (2006) The management of incisional hernia. Ann R Coll Surg Engl 88(3):252–260. https://doi.org/10.1308/003588406X106324. (PMID:16719992;PMCID:PMC1963672)
Baucom RB, Ousley J, Beveridge GB et al (2016) Cancer survivorship: defining the incidence of incisional hernia after resection for intra-abdominal malignancy. Ann Surg Oncol 23:764–771
Gignoux B, Bayon Y, Martin D, Phan R, Augusto V, Darnis B, Sarazin M (2021) Incidence and risk factors for incisional hernia and recurrence: retrospective analysis of the French national database. Colorectal Dis 23(6):1515–1523. https://doi.org/10.1111/codi.15581. (PMID: 33570808)
Bosanquet DC, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A et al (2015) Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14,618 patients. PLoS One 10(9):e0138745. https://doi.org/10.1371/journal.pone.0138745
Bhagwandin SB, Naffouje S, Salti G (2015) Delayed presentation of major complications in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy following hospital discharge. J Surg Oncol 111(3):324–327. https://doi.org/10.1002/jso.23834. (PMID: 25557653)
Chua TC, Yan T, Saxena A, Morris DL (2009) Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann Surg 249(6):900–907. https://doi.org/10.1097/SLA.0b013e3181a45d86
Ravn S, Thaysen HV, Harsløf S, Sørensen MM, Iversen LH (2018) Incisional hernia and its impact on health-related quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a national prospective cohort study. World J Surg Oncol 16(1):1–10
Struller F, Koenigsrainer I, Horvath P, Koenigsrainer A, Beckert S (2017) Abdominal wall morbidity following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Scand J Surg 106(4):294–298
Tuttle TM, Huang JL, Kizy S, Altman AM, Nalluri H, Marmor S et al (2019) Incidence and predictors of incisional hernia after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperth 36(1):811–815
Pamela K, Matthias Z, Reinhold KR, Julia P, Peter M, Alexander P, Dietmar Ö (2018) Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a single-center experience in Austria. J Gastrointest Surg 22(5):884–893. https://doi.org/10.1007/s11605-017-3661-1. (PMID: 29363016; PMCID: PMC5954007)
Ahn BK (2012) Risk factors for incisional hernia and parastomal hernia after colorectal surgery. J Korean Soc Coloproctol 28(6):280–1. https://doi.org/10.3393/jksc.2012.28.6.280. (PMID: 23346503; PMCID: PMC3548139)
Neuwirth MG, Alexander HR, Karakousis GC (2016) Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol 7(1):18–28. https://doi.org/10.3978/j.issn.2078-6891.2015.106. (PMID:26941981;PMCID:PMC4754315)
Itatsu K et al (2014) Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg 101(11):1439–1447
Wenzelberg C et al (2023) Abdominal closure with reinforcing suture decreases incisional hernia incidence after CRS/HIPEC. J Abdom Wall Surg. https://doi.org/10.3389/jaws.2023.11188
Wenzelberg C, Petersson U, Syk I, Ekberg O, Rogmark P (2023) Abdominal closure with reinforcing suture decreases incisional hernia incidence after CRS/HIPEC. J Abdom Wall Surg. https://doi.org/10.3389/jaws.2023.11188
Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J (2018) Clinical studies in CRS and HIPEC: trials, tribulations, and future directions-A systematic review. J Surg Oncol 117(2):245–259. https://doi.org/10.1002/jso.24813. (PMID: 29120491; PMCID: PMC6692902)
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol 134:178–189
Brown D (2020) A review of the PubMed PICO Tool: using evidence-based practice in health education. Health Promot Pract 21(4):496–498
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—A web and mobile app for systematic reviews. Syst Rev 5(1):210
Parikh R, Shah S, Dhurandhar V, Alzahrani N, Fisher OM, Arrowaili A et al (2019) An analysis of the morbidity associated with abdominal wall resection and reconstruction after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). Eur J Surg Oncol 45(3):394–399
Spencer RJ, Hayes KD, Rose S, Zhao Q, Rathouz PJ, Rice LW et al (2015) Risk factors for early and late-occurring incisional hernias after primary laparotomy for ovarian cancer. Obstet Gynecol 125(2):407
Wong J, Koch AL, Deneve JL, Fulp W, Tanvetyanon T, Dessureault S (2014) Repeat cytoreductive surgery and heated intraperitoneal chemotherapy may offer survival benefit for intraperitoneal mesothelioma: a single institution experience. Ann Surg Oncol 21:1480–1486
Wells G, Shea B, O’Connell D, Peterson j, Welch V, Losos M, et al. (2000) The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis
Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E et al (2021) Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol 21(1):189
Nyaga VN, Arbyn M, Aerts M (2014) Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 72(1):39
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48
Lin L, Chu H (2020) Meta-analysis of proportions using generalized linear mixed models. Epidemiology 31(5):713–717
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):135
Lewcun JA, Pauli EM, Pameijer C (2020) Incisional hernia formation can be reduced following hyperthermic intraperitoneal chemotherapy with increased suture length to wound length ratio fascial closure. Int J Abdom Wall Hernia Surg 3(4):117
Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A et al (2014) Incisional hernia rate 3 years after midline laparotomy. Br J Surg 101(2):51–54
Kohler A, Lavanchy JL, Lenoir U, Kurmann A, Candinas D, Beldi G (2019) Effectiveness of prophylactic intraperitoneal mesh implantation for prevention of incisional hernia in patients undergoing open abdominal surgery: a randomized clinical trial. JAMA Surg 154(2):109–115
O’Dwyer PJ, Courtney CA (2003) Factors involved in abdominal wall closure and subsequent incisional hernia. Surgeon 1(1):17–22
Höer J, Lawong G, Klinge U, Schumpelick V (2002) Factors influencing the development of incisional hernia. A retrospective study of 2983 laparotomy patients over a period of 10 years. Chirurg 73(5):474–80
Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J et al (2015) Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 386(10000):1254–1260
Millbourn D, Cengiz Y, Israelsson LA (2009) Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg 144(11):1056–1059
Beadles CA, Meagher AD, Charles AG (2015) Trends in emergent hernia repair in the United States. JAMA Surg 150(3):194–200
Wehrle CJ, Shukla P, Miller BT, Blake KE, Prabhu AS, Petro CC et al (2023) Incisional hernia rates following midline laparotomy in the obese patient: a retrospective review. Hernia 27(3):557–563
Sneiders D, Yurtkap Y, Kroese LF, Kleinrensink GJ, Lange JF, Gillion JF (2019) Risk factors for incarceration in patients with primary abdominal wall and incisional hernias: a prospective study in 4472 patients. World J Surg 43(8):1906–1913
Davila DG, Parikh N, Frelich MJ, Goldblatt MI (2016) The increased cost of ventral hernia recurrence: a cost analysis. Hernia 20(6):811–817
Shah DK, Patel SJ, Chaudhary SR, Desai NR (2023) Comparative study of onlay versus sublay mesh repair in the management of ventral hernias. Updates Surg. https://doi.org/10.1007/s13304-023-01532-5
Barranquero AG, Villalobos Mori R, Maestre González Y, Protti GP, López Soler G, Villarreal León F et al (2023) Parietex™ composite ventral patch for primary and incisional hernia repair. ANZ J Surg. https://doi.org/10.1111/ans.18524
Idiz C, Cakir C (2020) Nutritional status and constipation scoring of inguinal hernia patients: a case-control study. Hernia 24(5):1107–1112
Burnett A, Lecompte M-EA, Trabulsi N, Dubé P, Gervais M-K, Trilling B et al (2019) Peritoneal carcinomatosis index predicts survival in colorectal patients undergoing HIPEC using oxaliplatin: a retrospective single-arm cohort study. World J Surg Oncol 17(1):83
de Boer NL, Brandt-Kerkhof ARM, Madsen EVE, Doukas M, Verhoef C, Burger JWA (2021) The accuracy of the surgical peritoneal cancer index in patients with peritoneal metastases of colorectal cancer. Dig Surg 38(3):205–211
Kofoed NG, Falconer H, Vanky H, Johansson H, Abraham-Nordling M, Salehi S (2023) Survival and chance of reversal after intestinal stoma formation during cytoreductive surgery for advanced ovarian cancer; a population-based cohort study. Gynecol Oncol 170:259–265
Feenstra TM, Verberne CJ, Kok NF, Aalbers AGJ (2022) Anastomotic leakage after cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal cancer. Eur J Surg Oncol 48(12):2460–2466
Sugarbaker P (2018) Hernia mesh is contraindicated in patients with peritoneal metastases. JSM Surg Proced 1(1):1001
Tzivanakis A, Dayal SP, Arnold SJ, Mohamed F, Cecil TD, Venkatasubramaniam AK et al (2018) Biological mesh is a safe and effective method of abdominal wall reconstruction in cytoreductive surgery for peritoneal malignancy. BJS Open 2(6):464–469
Bhangu A, Fitzgerald JE, Singh P, Battersby N, Marriott P, Pinkney T (2013) Systematic review and meta-analysis of prophylactic mesh placement for prevention of incisional hernia following midline laparotomy. Hernia 17(4):445–455
Funding
Open Access funding provided by the IReL Consortium. This work did not receive funding from any source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval, Human and animal rights and Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mac Curtain, B.M., Qian, W., Temperley, H.C. et al. Incisional hernias post cytoreductive surgery/peritonectomy and hyperthermic intraperitoneal chemotherapy: a systematic review and meta-analysis. Hernia 27, 1067–1083 (2023). https://doi.org/10.1007/s10029-023-02859-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-023-02859-z