Abstract
Background
Infectious complications following mesh implantation for abdominal wall repair appear in 0.7 up to 26.6% of hernia repairs and can have a detrimental impact for the patient. To prevent or to treat mesh-related infection, the scientific community is currently developing a veritable arsenal of antibacterial meshes. The numerous and increasing reports published every year describing new technologies indicate a clear clinical need, and an academic interest in solving this problem. Nevertheless, to really appreciate, to challenge, to compare and to optimize the antibacterial properties of next generation meshes, it is important to know which models are available and to understand them.
Purpose
We proposed for the first time, a complete overview focusing only on the in vitro and in vivo models which have been employed specifically in the field of antibacterial meshes for hernia repair.
Results and conclusion
From this investigation, it is clear that there has been vast progress and breadth in new technologies and models to test them. However, it also shows that standardization or adoption of a more restricted number of models would improve comparability and be a benefit to the field of study.

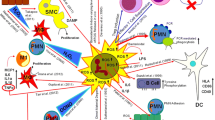
Adapted with permission from [40]

Similar content being viewed by others
References
Guillaume O, Teuschl AH, Gruber-Blum S, Fortelny RH, Redl H, Petter-Puchner A (2015) Emerging trends in abdominal wall reinforcement: bringing bio-functionality to meshes. Adv Healthc Mater 4(12):1763–1789
Guillaume O, Perez-Tanoira R, Fortelny R, Redl H, Moriarty TF, Richards RG, Eglin D, Petter Puchner A (2018) Infections associated with mesh repairs of abdominal wall hernias: are antimicrobial biomaterials the longed-for solution? Biomaterials 167:15–31
Perez-Kohler B, Bayon Y, Bellon JM (2016) Mesh infection and hernia repair: a review. Surg Infect (Larchmt) 17(2):124–137
Vogels RRM, Kaufmann R, van den Hil LCL, van Steensel S, Schreinemacher MHF, Lange JF, Bouvy ND (2017) Critical overview of all available animal models for abdominal wall hernia research. Hernia 21(5):667–675
The European Committee on antimicrobial susceptibility testing (EUCAST, 2017). Antimicrobial susceptibility testing: EUCAST disk diffusion method , Version 6.0. http://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/
Fernandez-Gutierrez M, Olivares E, Pascual G, Bellon JM, San Roman J (2013) Low-density polypropylene meshes coated with resorbable and biocompatible hydrophilic polymers as controlled release agents of antibiotics. Acta Biomater 9(4):6006–6018
Hong Y, Fujimoto K, Hashizume R, Guan J, Stankus JJ, Tobita K, Wagner WR (2008) Generating elastic, biodegradable polyurethane/poly(lactide-co-glycolide) fibrous sheets with controlled antibiotic release via two-stream electrospinning. Biomacromolecules 9(4):1200–1207
Guillaume O, Garric X, Lavigne JP, Van Den Berghe H, Coudane J (2012) Multilayer, degradable coating as a carrier for the sustained release of antibiotics: preparation and antimicrobial efficacy in vitro. J Control Release 162(3):492–501
Laurent T, Kacem I, Blanchemain N, Cazaux F, Neut C, Hildebrand HF, Martel B (2011) Cyclodextrin and maltodextrin finishing of a polypropylene abdominal wall implant for the prolonged delivery of ciprofloxacin. Acta Biomater 7(8):3141–3149
Junge K, Rosch R, Klinge U, Krones C, Klosterhalfen B, Mertens PR, Lynen P, Kunz D, Preiss A, Peltroche-Llacsahuanga H, Schumpelick V (2005) Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 26(7):787–793
Reslinski A, Dabrowiecki S, Glowacka K (2015) The impact of diclofenac and ibuprofen on biofilm formation on the surface of polypropylene mesh. Hernia 19(2):179–185
Engelsman AF, van der Mei HC, Busscher HJ, Ploeg RJ (2008) Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br J Surg 95(8):1051–1059
Majumder A, Scott JR, Novitsky YW (2016) Evaluation of the antimicrobial efficacy of a novel rifampin/minocycline-coated, noncrosslinked porcine acellular dermal matrix compared with uncoated scaffolds for soft tissue repair. Surg Innov 23(5):442–455
Satishkumar R, Sankar S, Yurko Y, Lincourt A, Shipp J, Heniford BT, Vertegel A (2011) Evaluation of the antimicrobial activity of lysostaphin-coated hernia repair meshes. Antimicrob Agents Chemother 55(9):4379–4385
Guillaume O, Lavigne JP, Lefranc O, Nottelet B, Coudane J, Garric X (2011) New antibiotic-eluting mesh used for soft tissue reinforcement. Acta Biomater 7(9):3390–3397
Klink CD, Binnebosel M, Lambertz A, Alizai HP, Roeth A, Otto J, Klinge U, Neumann UP, Junge K (2012) In vitro and in vivo characteristics of gentamicin-supplemented polyvinylidenfluoride mesh materials. J Biomed Mater Res A 100(5):1195–1202
Yurko Y, McDeavitt K, Kumar RS, Martin T, Prabhu A, Lincourt AE, Vertegel A, Heniford BT (2012) Antibacterial mesh: a novel technique involving naturally occurring cellular proteins. Surg Innov 19(1):20–26
Chen J, Howell C, Haller CA, Patel MS, Ayala P, Moravec KA, Dai E, Liu L, Sotiri I, Aizenberg M, Aizenberg J, Chaikof EL (2017) An immobilized liquid interface prevents device associated bacterial infection in vivo. Biomaterials 113:80–92
Demirer S, Gecim IE, Aydinuraz K, Ataoglu H, Yerdel MA, Kuterdem E (2001) Affinity of Staphylococcus epidermidis to various prosthetic graft materials. J Surg Res 99(1):70–74
Badiou W, Lavigne JP, Bousquet PJ, O’Callaghan D, Mares P, de Tayrac R (2011) In vitro and in vivo assessment of silver-coated polypropylene mesh to prevent infection in a rat model. Int Urogynecol J 22(3):265–272
Letouzey V, Lavigne JP, Garric X, Coudane J, de Tayrac R, Callaghan DO (2012) Is degradable antibiotic coating for synthetic meshes provide protection against experimental animal infection after fascia repair? J Biomed Mater Res B Appl Biomater 100(2):471–479
Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen MJ (2010) Novel in vitro model for assessing susceptibility of synthetic hernia repair meshes to Staphylococcus aureus infection using green fluorescent protein-labeled bacteria and modern imaging techniques. Surg Infect (Larchmt) 11(5):449–454
Stoodley P, Sidhu S, Nistico L, Mather M, Boucek A, Hall-Stoodley L, Kathju S (2012) Kinetics and morphology of polymicrobial biofilm formation on polypropylene mesh. FEMS Immunol Med Microbiol 65(2):283–290
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Grafmiller KT, Zuckerman ST, Petro C, Liu L, von Recum HA, Rosen MJ, Korley JN (2016) Antibiotic-releasing microspheres prevent mesh infection in vivo. J Surg Res 206(1):41–47
Harth KC, Rosen MJ, Thatiparti TR, Jacobs MR, Halaweish I, Bajaksouzian S, Furlan J, von Recum HA (2010) Antibiotic-releasing mesh coating to reduce prosthetic sepsis: an in vivo study. J Surg Res 163(2):337–343
Engelsman AF, van Dam GM, van der Mei HC, Busscher HJ, Ploeg RJ (2010) In vivo evaluation of bacterial infection involving morphologically different surgical meshes. Ann Surg 251(1):133–137
Poelstra KA, Barekzi NA, Rediske AM, Felts AG, Slunt JB, Grainger DW (2002) Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res 60(1):206–215
Bhende S, Barbolt T, Rothenburger S, Piccoli L (2007) Infection potentiation study of synthetic and naturally derived surgical mesh in mice. Surg Infect (Larchmt) 8(3):405–414
Bellows CF, Wheatley BM, Moroz K, Rosales SC, Morici LA (2011) The effect of bacterial infection on the biomechanical properties of biological mesh in a rat model. PLoS One 6(6):e21228
Belyansky I, Tsirline VB, Martin TR, Klima DA, Heath J, Lincourt AE, Satishkumar R, Vertegel A, Heniford BT (2011) The addition of lysostaphin dramatically improves survival, protects porcine biomesh from infection, and improves graft tensile shear strength. J Surg Res 171(2):409–415
Belyansky I, Tsirline VB, Montero PN, Satishkumar R, Martin TR, Lincourt AE, Shipp JI, Vertegel A, Heniford BT (2011) Lysostaphin-coated mesh prevents staphylococcal infection and significantly improves survival in a contaminated surgical field. Am Surg 77(8):1025–1031
Bury K, Smietanski M, Justyna B, Gumiela P, Smietanska AI, Owczuk R, Naumiuk L, Samet A, Paradziej-Lukowicz J (2014) Effects of macroporous monofilament mesh on infection in a contaminated field. Langenbecks Arch Surg 399(7):873–877
Sadava EE, Krpata DM, Gao Y, Novitsky YW, Rosen MJ (2013) Does presoaking synthetic mesh in antibiotic solution reduce mesh infections? An experimental study. J Gastrointest Surg 17(3):562–568
Cole WC, Balent EM, Masella PC, Kajiura LN, Matsumoto KW, Pierce LM (2015) An experimental comparison of the effects of bacterial colonization on biologic and synthetic meshes. Hernia 19(2):197–205
Deerenberg EB, Mulder IM, Grotenhuis N, Ditzel M, Jeekel J, Lange JF (2012) Experimental study on synthetic and biological mesh implantation in a contaminated environment. Br J Surg 99(12):1734–1741
Suárez-Grau JM, Morales-Conde S, González Galán V, Martín Cartes JA, Docobo Durantez F, Padillo Ruiz FJ (2015) Antibiotic embedded absorbable prosthesis for prevention of surgical mesh infection: experimental study in rats. Hernia 19(2):187–194
Zhou HY, Zhang J, Yan RL, Wang Q, Fan LY, Zhang Q, Wang WJ, Hu ZQ (2011) Improving the antibacterial property of porcine small intestinal submucosa by nano-silver supplementation: a promising biological material to address the need for contaminated defect repair. Ann Surg 253(5):1033–1041
Harth KC, Broome AM, Jacobs MR, Blatnik JA, Zeinali F, Bajaksouzian S, Rosen MJ (2011) Bacterial clearance of biologic grafts used in hernia repair: an experimental study. Surg Endosc 25(7):2224–2229
Harth KC, Blatnik JA, Anderson JM, Jacobs MR, Zeinali F, Rosen MJ (2013) Effect of surgical wound classification on biologic graft performance in complex hernia repair: an experimental study. Surgery 153(4):481–492
Cakmak A, Cirpanli Y, Bilensoy E, Yorganci K, Calis S, Saribas Z, Kaynaroglu V (2009) Antibacterial activity of triclosan chitosan coated graft on hernia graft infection model. Int J Pharm 381(2):214–219
Ott R, Hartwig T, Tannapfel A, Blatz R, Rodloff AC, Madaj-Sterba P, Mobius C, Kockerling F (2007) Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg 392(4):473–478
Saygun O, Agalar C, Aydinuraz K, Agalar F, Daphan C, Saygun M, Ceken S, Akkus A, Denkbas EB (2006) Gold and gold-palladium coated polypropylene grafts in a S. epidermidis wound infection model. J Surg Res 131(1):73–79
Pérez-Tanoira R, Lévano-Linares C, Celdrán-Uriarte Á, Isea-Peña MC, De Molina MS, García-Vasquez C, Esteban-Moreno J (2016) Use of an experimental model to evaluate infection resistance of meshes in abdominal wall surgery. J Surg Res 206(2):435–441
Carbonell AM, Matthews BD, Dreau D, Foster M, Austin CE, Kercher KW, Sing RF, Heniford BT (2005) The susceptibility of prosthetic biomaterials to infection. Surg Endosc 19(3):430–435
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63(6):765–771
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ (2012) In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg 16(11):2139–2144
Medberry CJ, Tottey S, Jiang HB, Johnson SA, Badylak SF (2012) Resistance to infection of five different materials in a rat body wall model. J Surg Res 173(1):38–44
Mulder IM, Deerenberg EB, Bemelman WA, Jeekel J, Lange JF (2015) Infection susceptibility of crosslinked and non-crosslinked biological meshes in an experimental contaminated environment. Am J Surg 210(1):159–166
Kaufmann R, Jairam AP, Mulder IM, Wu Z, Verhelst J, Vennix S, Giesen LJX, Clahsen-van MC, Groningen J, Jeekel JF, Lange (2017) Characteristics of different mesh types for abdominal wall repair in an experimental model of peritonitis. Br J Surg 104:1884–1893
Bellon JM, Garcia-Carranza A, Garcia-Honduvilla N, Carrera-San Martin A, Bujan J (2004) Tissue integration and biomechanical behaviour of contaminated experimental polypropylene and expanded polytetrafluoroethylene implants. Br J Surg 91(4):489–494
Bellon JM, Contreras LA, Bujan J (2000) Ultrastructural alterations of polytetrafluoroethylene prostheses implanted in abdominal wall provoked by infection: clinical and experimental study. World J Surg 24(5):528–531
Garcia-Pumarino R, Pascual G, Rodriguez M, Perez-Kohler B, Bellon JM (2014) Do collagen meshes offer any benefits over preclude (R) ePTFE implants in contaminated surgical fields? A comparative in vitro and in vivo study. J Biomed Mater Res Part B Appl Biomater 102(2):366–375
Perez-Kohler B, Garcia-Moreno F, Brune T, Pascual G, Bellon JM (2015) Preclinical bioassay of a polypropylene mesh for hernia repair pretreated with antibacterial solutions of chlorhexidine and allicin: an in vivo study. PLoS One 10(11):e0142768
Goeau-Brissonniere O, Leflon V, Letort M, Nicolas MH (1999) Resistance of antibiotic-bonded gelatin-coated polymer meshes to Staphylococcus aureus in a rabbit subcutaneous pouch model. Biomaterials 20(3):229–232
Diaz-Godoy A, Garcia-Urena MA, Lopez-Monclus J, Ruiz VV, Montes DM, Agurto NE (2011) Searching for the best polypropylene mesh to be used in bowel contamination. Hernia 15(2):173–179
Fernandez-Moure JS, Van Eps JL, Peress L, Cantu C, Olsen RJ, Jenkins L, Cabrera FJ, Tasciotti E, Weiner BK, Dunkin BJ (2017) Increased use of surgical energy promotes methicillin-resistant Staphylococcus aureus colonization in rabbits following open ventral hernia mesh repair. Surg Endosc 31(2):852–860
Stoikes NFN, Scott JR, Badhwar A, Deeken CR, Voeller GR (2017) Characterization of host response, resorption, and strength properties, and performance in the presence of bacteria for fully absorbable biomaterials for soft tissue repair. Hernia 21(5):771–782
Smith S, Gantt N, Rowe MI, Lloyd DA (1989) Dura versus gore-tex as an abdominal wall prosthesis in an open and closed infected model. J Pediatr Surg 24(6):519–521
Brown GL, Richardson JD, Malangoni MA, Tobin GR, Ackerman D, Polk HC (1985) Comparison of prosthetic materials for abdominal wall reconstruction in the presence of contamination and infection. Ann Surg 201(6):705–711
Blatnik JA, Thatiparti TR, Krpata DM, Zuckerman ST, Rosen MJ, von Recum HA (2017) Infection prevention using affinity polymer-coated, synthetic meshes in a pig hernia model. J Surg Res 219:5–10
Krizek TJ, Robson MC (1975) Evolution of quantitative bacteriology in wound management. Am J Surg 130(5):579–584
Buer J, Balling R (2003) Mice, microbes and models of infection. Nat Rev Genet 4(3):195–205
Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJ, Zaat SA, Schultz MJ, Grainger DW (2012) Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med 4(153):153rv10
Bryda EC (2013) The mighty mouse: the impact of rodents on advances in biomedical research. Mol Med 110(3):207–211
Suckow MA, Stevens KA, Wilson RP (2012) The laboratory rabbit, guinea pig, hamster, and other rodents. A volume in American College of Laboratory Animal Medicine, Elsevier Inc. (Ed.), Amsterdam, pp 1–1268
Chemali JJ, Kenny JD, Olutola O, Taylor NE, Kimchi EY, Purdon PL, Brown EN, Solt K (2015) Ageing delays emergence from general anaesthesia in rats by increasing anaesthetic sensitivity in the brain. Br J Anaesth 115(Suppl 1):i58–i65
Martín-Zúñiga J (2014) Ciencia y tecnología en experimentación y protección animal. University of Alcalá, Spanish Association of Laboratory Animal Science (SECAL), University of Alcalá Health Science Textbooks, Alcalá de Henares, pp 1–969
Jensen LK, Johansen ASB, Jensen HE (2017) Porcine models of biofilm infections with focus on pathomorphology. Front Microbiol 8:1961
Padilla-Carlin DJ, McMurray DN, Hickey AJ (2008) The guinea pig as a model of infectious diseases. Comp Med 58(4):324–340
Majumder A, Petro CC, Liu L, Fayezizadeh M, Novitsky YW (2016) Development of a novel murine model for treatment of infected mesh scenarios. Surg Endosc 31:922–927
Petter-Puchner AH, Fortelny RH (2015) The heart of darkness. Hernia 19(2):195–196
Kathju S, Nistico L, Melton-Kreft R, Lasko LA, Stoodley P (2015) Direct demonstration of bacterial biofilms on prosthetic mesh after ventral herniorrhaphy. Surg Infect (Larchmt) 16(1):45–53
Majumder A, Neupane R, Novitsky YW (2015) Antibiotic coating of hernia meshes: the next step toward preventing mesh infection. Surg Technol Int 27:147–153
Perez-Kohler B, Sotomayor S, Rodriguez M, Gegundez MI, Pascual G, Bellon JM (2015) Bacterial adhesion to biological versus polymer prosthetic materials used in abdominal wall defect repair: do these meshes show any differences in vitro? Hernia 19(6):965–973
Celdrán A, Esteban J, Mañas J, Granizo JJ (2007) Wound infections due to Mycobacterium fortuitum after polypropylene mesh inguinal hernia repair. J Hosp Infect 66(4):374–377
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Ethical approval
Approval from the institutional review board was not required for this study.
Human and animal rights
This article does not contain any studies with human participants or animals peformed by any of the authors.
Informed consent
For this literature review, formal consent is not required.
Rights and permissions
About this article
Cite this article
Guillaume, O., Pérez Kohler, B., Fortelny, R. et al. A critical review of the in vitro and in vivo models for the evaluation of anti-infective meshes. Hernia 22, 961–974 (2018). https://doi.org/10.1007/s10029-018-1807-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-018-1807-z