Abstract
Purpose
This study aimed to determine the outcome of perineal hernia repair with a biological mesh after abdominoperineal resection (APR).
Method
All consecutive patients who underwent perineal hernia repair with a porcine acellular dermal mesh between 2010 and 2014 were included. Follow-up was performed by clinical examination and MRI.
Results
Fifteen patients underwent perineal hernia repair after a median of 25 months from APR. Four patients had a concomitant contaminated perineal defect, for which a gluteal fasciocutaneous flap was added in three patients. Wound infection occurred in three patients. After a median follow-up of 17 months (IQR 12–24), a clinically recurrent perineal hernia developed in 7 patients (47 %): 6 of 11 patients after a non-cross-linked mesh and 1 of 4 patients after a cross-linked mesh (p = 0.57). Routine MRI at a median of 17 months revealed a recurrent perineal hernia in 7 of 10 evaluable patients, with clinical confirmation of recurrence in 5 of these 7 patients. No recurrent hernia was observed in the three patients with combined flap reconstruction for contaminated perineal defects.
Conclusion
A high recurrence rate was observed after biological mesh repair of a perineal hernia following APR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reported incidence of perineal hernia ranges between 1 and 13 % after abdominoperineal resection (APR) with primary perineal wound closure for rectal cancer [1]. True incidences of perineal hernia might even be higher because of the underreporting of (asymptomatic) perineal hernia. In addition, literature data may not be representative for current practice with the increasing use of the extralevator APR, which has been associated with perineal hernia rate of up to 26 % [2].
Patients with a symptomatic perineal hernia most often present with perineal discomfort, pain and urinary dysfunction, and rarely with bowel obstruction. Treatment of perineal hernia is most often conservative, consisting of supportive undergarments. When surgical intervention is indicated, mesh repair is preferred over primary suturing as a basic treatment principle in hernia surgery [3].
Several types of meshes have been used for perineal hernia repair, including biological meshes [3]. Biological meshes are suitable for contaminated fields and are supposed to result in fewer bowel adhesions compared to synthetic meshes [4, 5]. Because of these characteristics, a biological mesh may be considered to be of additional value in the potentially contaminated area of the perineum and the possibility of small bowel lying on top of it. However, current literature on perineal hernia repair with a biological mesh mainly consists of case reports. Therefore, the aim of this study was to determine the postoperative outcome and mid-term follow-up, including magnetic resonance imaging (MRI), of a consecutive cohort of perineal hernia repair using a biological mesh.
Patients and methods
All consecutive patients who underwent a perineal hernia repair between March 2010 and April 2014 at the Academic Medical Centre, Amsterdam were included. All perineal hernia reconstructions were performed or supervised by two colorectal surgeons (PT and WB) and performed in prone position using a transperineal approach, except for one patient who underwent a transabdominal approach. Transperineal repair started with resection of the redundant skin and hernia sac. Subsequently, a porcine acellular dermal mesh was sutured to the sacrococcygeal ligaments dorsally with interrupted polypropylene 2.0 sutures, resulting in about 2 cm overlap of the mesh to the sacrum. Laterally, the mesh was fixated to the remnants of the levator muscle with a small overlap of about 0.5–1.5 cm, and anteriorly the mesh was folded with about 2 cm overlap against the posterior vaginal wall or prostate and sutured to the transverse perineal muscle (Fig. 1). During the study period, we switched from a cross-linked biological mesh (Permacol™ 10 × 10 cm) to a non-cross-linked mesh (Strattice™ 6 × 10 cm), related to institutional uniformity in the use of a biological mesh for all indications. Thereafter, a 10 French vacuum drain was placed on top of the mesh and the subcutaneous tissue and skin were closed. In most cases, the mesh was covered top side with omentum because an omentoplasty was performed at the index operation as a routine. When no omentoplasty was performed at the index operation, a transabdominal laparoscopic omentoplasty was performed during perineal hernia repair. If there was insufficient soft tissue to cover the mesh bottom side, a gluteal fasciocutaneous transposition flap was used for perineal closure.
Postoperatively, the drain was removed after 3–7 days. Patients were fully mobilized at postoperative day one, except for those with a gluteal fasciocutaneous flap, who were mobilized after 3 days and were allowed to sit after 7 days. Follow-up was at least 12 months and consisted of clinical examination during each visit to the outpatient clinic. In addition, 10 patients underwent MRI as routine follow-up between 7 and 41 months from perineal hernia repair. MRI was performed on 1.5–3 Tesla scanners (Philips Healthcare, Best, the Netherlands and Siemens Avanto, Erlangen, Germany) with an axial and/or sagittal, coronal T2-weighted sequence, and a sagittal and/or coronal dynamic sequence with Valsalva. The MRI was evaluated by a gastrointestinal radiologist with extensive experience in pelvic MRI (JS).
Data extraction
Patient records were retrospectively searched for patient and treatment characteristics. Baseline data were extracted on underlying disease, (chemo) radiotherapy, extent of primary APR, the use of an omentoplasty and the method of perineal wound closure. Operative reports of perineal hernia repair were searched for the description of the operative approach (transperineal, transabdominal), the type of biological mesh used (cross-linked, non-cross-linked), and the use of an additional gluteal transposition flap. Outcome parameters were hospital stay, perineal wound infection, clinical and radiological recurrence of perineal hernia, and perineal re-interventions. Clinical recurrent hernia was defined as a midline swelling in standing position with absence of the anal cleft and a palpable pelvic floor defect. A radiological hernia was defined as descent of small bowel or omentoplasty below the line between the coccyx and the perineal body (Fig. 3) or below visible remnants of a mesh.
Statistical analysis
According to the distribution, descriptive data were reported as median with interquartile range (IQR) or mean ± standard deviation (SD). Categorical data were analyzed with the Chi square test or Fisher’s exact test. All analyses were performed with IBM SPSS statistics, version 20.0.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Between March 2010 and April 2014, 15 patients were operated for a perineal hernia after APR. The patient characteristics are displayed in Table 1. An extralevator approach was performed in five of 11 rectal cancer patients, but in none of them the coccyx was removed. The perineum was closed primarily in 13 patients, closed with a biological mesh (Permacol™) in one patient, and primary Vacuum Assisted Closure (VAC) was applied in the remaining patient. A postoperative perineal wound infection occurred in four of 14 patients with intentionally primary wound healing, of whom one underwent reoperation for a presacral abscess and one was treated with VAC therapy.
A symptomatic perineal hernia developed after a median of 14 months (IQR 5–34). A total of five patients were referred from another hospital. Two of them underwent surgical intervention prior to referral. One patient underwent laparoscopic biological mesh (Permacol™) repair of a perineal hernia 13 months after APR, followed by hysterectomy with McCall culdoplasty 17 months later, and developed a recurrent perineal hernia. The other patient underwent hysterectomy and sacrocolpopexy using a prolene mesh (gynemesh®) after 18 months because of sexual dysfunction and feeling of pressure in the perineum and developed recurrent symptoms with radiological descent of small bowel beyond the coccyx on imaging.
Perineal hernia repair
Primary perineal hernia repair was performed in 14 patients after a median of 25 months (IQR 17–55) from primary APR. The remaining patient was operated upon for recurrent perineal hernia 51 months from primary APR and 38 months from first perineal hernia repair. Three female patients had a non-healing perineal wound together with perineal herniation, and one male patient was admitted from the emergency department with a perineal necrosis and infection. n = 3 primary cross linked mesh reconstruction at the AMC; n = 11 primary non-cross linked mesh reconstruction at the AMC; n = 1 referral to the AMC after cross-linked mesh reconstruction at referring centre; secondary repair using non-cross linked mesh after removal of the cross-linked mesh at the AMC. In the remaining patient, the ‘meshoma’ consisting of a detached and encapsulated cross-linked biological mesh was removed (Fig. 2) and a new reconstruction of the pelvic floor was performed using a non-cross-linked biological mesh. Details of the perineal hernia and the surgical repair are summarized in Table 2. The median duration of the operation without the gluteal fasciocutaneous flap was 97 min (IQR 73–134), which was significantly longer than 231 min (IQR 206–231) when a gluteal fasciocutaneous flap was performed (p = 0.016). A postoperative perineal wound infection requiring antibiotic therapy occurred in three patients, with percutaneous drainage in one of them. No seroma or fistula formation was observed. The median postoperative hospital stay was three days (IQR 2–6).
Outcome of perineal hernia reconstruction
The overall clinical recurrence rate of primary perineal hernia repair using a biological mesh, including the patient who underwent initial repair at the referring hospital, was seven of 15 patients (47 %). Recurrent perineal hernia was diagnosed after a median of 17 months (IQR 12–24). The median follow-up after hernia repair in patients not having a recurrent perineal hernia was 20 months (IQR 13–44). A recurrent perineal hernia occurred in six out of 11 non-cross-linked biological mesh repairs, and in one out of four primary repairs using a cross-linked biological mesh (p = 0.57). None of the three patients treated with an additional gluteal fasciocutaneous flap had a recurrent perineal hernia after 13, 13 and 21 months of follow-up.
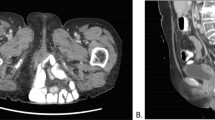
Routine MRI was performed in 10 patients after a median of 17 months (IQR 11–30) from perineal hernia repair (Fig. 3). Five patients did not undergo MRI for the following reasons; palliative setting with metastatic disease in two patients, short follow-up (<6 months) in two patients, and a pacemaker in one patient. Nine out of 10 patients had perineal complaints at the time of MRI, and a clinical recurrent hernia was diagnosed by physical examination in five of these patients. The main complaints consisted of a pressing sensation in seven patients, of perineal pain in two patients, and dyspareunia in the remaining patient. A radiological recurrent perineal hernia was diagnosed on MRI in seven out of the 10 patients. In one of these seven patients, herniation along both lateral borders of a cross-linked mesh was visible after 41 months, but herniation could not be confirmed clinically. In the other six patients, remnants of a non-cross-linked biological mesh could be identified after an interval between 12 and 28 months (Fig. 3b, c), with radiological herniation in all these patients and clinical recurrence in five patients. In the three other patients, an intact cross-linked mesh was visible after 36 months, and an intact non-cross-linked biological mesh was visible at 7 and 10 months postoperatively without signs of recurrent hernia (Fig. 3a). Of the seven patients with a recurrent hernia, four patients underwent a redo perineal hernia reconstruction with a synthetic mesh (Table 2). The sutures of the previous reconstruction were not migrated, and the initial non-cross-linked biological mesh could not be identified anymore.
MRI images of two patients after perineal hernia repair. a Sagittal plane, T2-weighted sequence, sufficient hernia repair with visible biological mesh between the coccyx and the perineal body. b, c Transverse and sagittal images, T2-weighted sequence, of a patient with a recurrent hernia, revealing remnants of the biological mesh along the posterior and right lateral borders of the perineal defect. Recurrent perineal hernia is defined as descent of small bowel or omentoplasty below the line drawn on a sagittal imaging. Arrow remnants of the biological mesh
Discussion
To our knowledge, we report the largest cohort of patients who underwent a biological mesh reconstruction of a perineal hernia. A relatively high recurrence rate of 47 % was found, which may even become higher with extended follow-up. Current literature on perineal hernia repair after APR is limited. A systematic review of the literature identified 39 relevant papers between 1939 and 2011, describing only 76 patients who underwent surgical repair of a perineal hernia [3]. A biological mesh was used in only five of those patients, reported by three different authors [6–8]. Only one case report on biological mesh repair has been published thereafter [9]. These case reports are mainly focused on surgical technique, have an inherent risk of publication bias with often reporting successes, and follow-up is insufficient to draw any conclusion on long-term outcome.
The 47 % recurrence rate found in our study is considerably higher than a recently reported 5 % recurrence rate after transperineal synthetic mesh reconstruction of uncomplicated perineal hernia repair in 21 patients with a median follow-up of 24 months [10]. Reported mesh size in case reports using synthetic meshes is also larger than the size of the non-cross-linked biological mesh that we used, with a mesh width ranging from 8 to 18 cm [9, 11–15]. Therefore, we recently changed our approach to synthetic mesh repair using a larger size (15 × 15 cm) for primary and recurrent perineal hernias in the absence of contamination, combined with an omentoplasty if not already present. We still prefer the transperineal approach, but others effectively use a (laparoscopic) trans-abdominal route of mesh placement [16, 17]. Reasons for using a transperineal approach are the easier ventral fixation to the transverse perineal muscles, the better visualization of the neurovascular bundles along the prostate that should not be included in the stitches to prevent postoperative pain, and the lesser costs compared to a laparoscopic approach with the use of disposables.
The 93 % radiotherapy rate may have contributed to the high failure rate. A biological mesh provides a scaffold for ingrowth by host tissue, leading to integration of the mesh [18]. Radiotherapy disrupts cellular cytokine reactions and reduces nitric oxide and metalloproteinase [19, 20]. As a result, inadequate soft tissue regeneration and disorganized deposition of collagen occur [19, 21]. The amount of cross linking might be another explanation for the high recurrence rate, because a non-cross-linked biological mesh was used in the majority of patients [22]. Cross-links are covalent bonds between the collagen and are supposed to resist collagen degradation by host or bacterial collagenase [23, 24]. Thereby, cross linking increases tensile strength which is supposed to result in a lower rate of recurrent hernia compared to non-cross-linked meshes, especially in a contaminated field. On the other hand, cross linking may restrict early cellular infiltration and may elicit an unintended inflammatory response, which could lead to fibrosis and could limit tissue remodeling [25, 26]. Encapsulation of a cross-linked mesh similar to a synthetic mesh was observed in one of our patients. Furthermore, fistula formation after a cross-linked biological mesh repair of a perineal hernia has been described [27]. Currently available literature does not allow for a definitive answer on the preferred type of biological mesh.
If no omentoplasty has been performed at time of primary APR, small bowel will lie on top of the mesh following perineal hernia repair. Direct contact of bowel loops to a mesh might lead to adhesion or fistula formation. Besides small bowel descent, an omentoplasty also minimizes dorsal displacement of the internal genital organs and bladder, which is associated with sexual and bladder dysfunction, respectively. This is the reason why we consider adding an omentoplasty to the hernia repair if not done so primarily to restore pelvic anatomy as much as possible.
In this study, hernia repair was combined with a gluteal fasciocutaneous flap (VY or SGAP) to fill and close a chronic perineal defect in three patients. A gluteal flap does probably not add any strength to the pelvic floor reconstruction [28]. However, it is important to adequately cover the mesh with well-vascularized subcutaneous tissue to prevent seroma and abscess formation below the mesh, and to close the perineal skin without tension. Loss of perineal tissue related to extended primary resection or infectious complications requiring debridement may require autologous tissue flap reconstruction. All three patients with combined biological mesh and flap reconstruction were still without signs of recurrent hernia at last follow-up. Probably, the use of a biological mesh for pelvic floor reconstruction should be restricted to such patients with contaminated perineal defects.
Adequate follow-up to determine success of hernia repair is essential and routine imaging may be the most objective measure. However, imaging might overestimate the clinically relevant recurrence rate as shown by the present data. MRI to evaluate perineal hernia repair using a biological mesh has first been described by Kavanagh et al. in a single case [29]. We performed MRI during follow-up to assess the basis for perineal complaints, with specific focus on mesh ingrowth and remodeling and potential mechanisms of technical failure. Because often little remnants of the mesh could be identified anymore on MRI, biological mesh degradation with inadequate tissue remodeling seemed to be the main reason for failure, which was supported by the findings during redo surgery. However, shrinkage of the biological mesh or the size of the mesh used might have contributed to the high recurrence rate as well [30]. With the standard available size of the non-cross-linked mesh for this indication (6 × 10 cm), there is only a few centimeters overlap ventrally and dorsally with almost no overlap towards the pelvic side walls. Furthermore, a transperineal approach does not allow for additional fixation of the edges of the overlapping mesh in addition to the sutures along the defect. This might be essential, because the mesh does often not smoothly follow the funnel shape of the pelvis, which limits contact of the mesh to surrounding tissues and may affect ingrowth.
Limitations of this study are its retrospective design and the limited number of patients included. Failure may be associated with restricted experience, because of the rarity of this problem. However, our group also conducted a multicenter randomized controlled trial on pelvic floor reconstruction using a biological mesh following extralevator APR during the study period, which contributed to our expertise in this field [31]. Also, follow-up is still relatively short, and imaging was not performed at standardized follow-up intervals.
Despite these limitations, this study shows that perineal hernia reconstruction with a 6 × 10 cm non-cross-linked biological mesh via a transperineal approach results in a high recurrence rate in patients who underwent APR for cancer. Our little experience with cross-linked biological mesh does not allow for any conclusion. A biological mesh may not be the first choice implant for perineal hernia repair in the absence of contamination and the presence of an omentoplasty.
References
Musters GD, Buskens CJ, Bemelamn WA, Tanis PJ (2014) Perineal wound healing after abdominoperineal resection for rectal cancer; a systematic review and meta-analysis. Dis Colon Rectum 57:1129–1139
Sayers AE, Patel RK, Hunter IA (2014) Perineal hernia formation following extralevator abdominoperineal excision. Colorectal Dis 17:351–355
Mjoli M, Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ (2012) Perineal hernia repair after abdominoperineal resection: a pooled analysis. Colorectal Dis 14(7):400–406
Burns NK, Jaffari MV, Rios CN, Mathur AB, Butler CE (2010) Non-cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg 125:167–176
Rosen MJ (2010) Biologic mesh for abdominal wall reconstruction: a critical appraisal. Am Surg 76:1–6
de Campos FG, Habr-Gama A, Araujo SE, Sousa AH Jr, Nahas CR, Lupinacci RM et al (2005) Incidence and management of perineal hernia after laparoscopic proctectomy. Surg Laparosc Endosc Percutan Tech 15:366–370
Kathju S, Lasko LA, Medich DS (2011) Perineal hernia repair with acellular dermal graft and suture anchor fixation. Hernia 15(3):357–360
Skipworth RJ, Smith GH, Anderson DN (2007) Secondary perineal hernia following open abdominoperineal excision of the rectum: report of a case and review of the literature. Hernia 11:541–545
Chelala E, Declercq S (2015) Laparoscopic repair of post-abdominoperineal resection hernia: biological mesh and augmentation technique. Hernia 19:853–856
Martijnse IS, Holman F, Nieuwenhuijzen GA, Rutten HJ, Nienhuijs SW (2012) Perineal hernia repair after abdominoperineal rectal excision. Dis Colon Rectum 55:90–95
Abdul Jabbar AS (2002) Postoperative perineal hernia. Hernia 6:188–190
Akatsu T, Murai S, Kamiya S, Kojima K, Mizuhashi Y, Hasegawa H et al (2009) Perineal hernia as a rare complication after laparoscopic abdominoperineal resection: report of a case. Surg Today 39:340–343
Frydman GM, Polglase AL (1989) Perineal approach for polypropylene mesh repair of perineal hernia. Aust N Z J Surg 59:895–897
Rayhanabad J, Sassani P, Abbas MA (2009) Laparoscopic repair of perineal hernia. JSLS 13:237–241
Ryan S, Kavanagh DO, Neary PC (2010) Laparoscopic repair of postoperative perineal hernia. Case Rep Med 9:2010
Abbas Y, Garner J (2014) Laparoscopic and perineal approaches to perineal hernia repair. Tech Coloproctol 18:361–364
Allen SK, Schwab K, Day A, Singh-Ranger D, Rockall TA (2014) Laparoscopic repair of post-operative perineal hernia using a two-mesh technique. Colorectal Dis 17:70–73
Novitsky YW (2013) Biology of biological meshes used in hernia repair. Surg Clin North Am 93:1211–1215
Gu Q, Wang D, Gao Y, Zhou J, Peng R, Cui Y et al (2002) Expression of MMP1 in surgical and radiation-impaired wound healing and its effects on the healing process. J Environ Pathol Toxicol Oncol 21:71–78
Herskind C, Bamberg M, Rodemann HP (1998) The role of cytokines in the development of normal-tissue reactions after radiotherapy. Strahlenther Onkol 174:12–15
Johnson LB, Jorgensen LN, Adawi D, Blomqvist P, Asklof GB, Gottrup F et al (2005) The effect of preoperative radiotherapy on systemic collagen deposition and postoperative infective complications in rectal cancer patients. Dis Colon Rectum 48:1573–1580
Deeken CR, Melman L, Jenkins ED, Greco SC, Frisella MM, Matthews BD (2011) Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg 212:880–888
de Castro Bras LE, Shurey S, Sibbons PD (2012) Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia 16:77–89
Oliver RF, Grant RA, Cox RW, Hulme MJ, Mudie A (1976) Histological studies of subcutaneous and intraperitoneal implants of trypsin-prepared dermal collagen allografts in the rat. Clin Orthop Relat Res 115:291–302
Cornwell KG, Landsman A, James KS (2009) Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 26:507–523
Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW (2004) Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 25:3541–3552
Eriksen M, Bulut O (2014) Chemotherapy-induced enterocutaneous fistula after perineal hernia repair using a biological mesh: a case report. Int Med Case Rep J 7:11–13
Christensen HK, Nerstrom P, Tei T, Laurberg S (2011) Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum 54:711–717
Kavanagh DO, Imran H, Almoudaris A, Ziprin P, Faiz O (2012) Dynamic magnetic resonance imaging demonstrates the integrity of perineal reconstruction following cylindrical abdominoperineal excision with reconstruction of the pelvic floor using porcine collagen. Case Rep Med 2012:752357
Mulder IM, Deerenberg EB, Bemelman WA, Jeekel J, Lange JF (2015) Infection susceptibility of crosslinked and non-crosslinked biological meshes in an experimental contaminated environment. Am J Surg 210(1):159–166
Musters GD, Bemelman WA, Bosker RJ, Burger JW et al (2014) Randomized controlled multicentre study comparing biological mesh closure of the pelvic floor with primary perineal wound closure after extralevator abdominoperineal resection for rectal cancer (BIOPEX-study). BMC Surg 14:58
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GM, OL, JS, BM declare no conflict of interest. WB and PT declare conflict of interest not directly related to the submitted work. The authors PT and WB have received a speaker honorarium from Lifecell company.
Funding
This study was not funded.
Ethical approval
The present study involved retrospective data collection, which does not need ethical approval according to the Dutch law.
Informed consent
Informed consent was obtained prior to treatment in all patients according to clinical practice, but no written informed consent has been obtained specifically for this retrospective study using anonymized data.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Musters, G.D., Lapid, O., Stoker, J. et al. Is there a place for a biological mesh in perineal hernia repair?. Hernia 20, 747–754 (2016). https://doi.org/10.1007/s10029-016-1504-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-016-1504-8