Abstract
Benthic macrofauna modifies carbon and nutrient retention and recycling processes in coastal habitats. However, the contribution of benthic consumers to carbon and nutrient storage and recycling shows variation over spatial scales, as the benthic community composition changes in response to differences in environmental conditions. By sampling both shallow sandy and deep muddy sediments across a land-to-sea gradient in the northern Baltic Sea, we explored if benthic community composition, stoichiometry and process rates change in response to alterations in environmental conditions and food sources. Our results show that benthic faunal biomass, C, N, and P stocks, respiration rate and secondary production increase across the land-to-sea gradient in response to higher resource quality towards the open sea. The seston δ13C indicated terrestrial runoff and δ15N sewage input at the innermost study sites, whereas more fresh marine organic matter towards the open sea boosted benthic faunal carbon storage, respiration rate, and secondary production, that is, the generation of consumer biomass, which are essential processes for carbon turnover in this coastal ecosystem. Also, biological factors such as increasing species richness and decreasing biomass dominance of the clam Macoma balthica were significant in predicting benthic faunal C, N, and P stocks and process rates, especially at sandy sites. Interestingly, despite the variation in food sources, the benthic faunal C:N:P ratios remained stable across the gradient. Our results prove that human activities in the coastal area can influence the important links between biodiversity, structure, and process rates of benthic communities by modifying the balance of available resources, therefore hampering the functioning of coastal ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Runoff from land and sewage input reduces food quality for benthic consumers
-
Increasing food quality enhances benthic community C, N, and P stocks and process rates
-
Changes in community composition alter carbon storage and turnover by benthic fauna
Introduction
Human activities are rapidly changing the availability and balance of carbon and nutrients in ecosystems on a global scale (Peñuelas and others 2019). This is alarming, as all organisms need these elements to maintain growth and reproduction for sustaining their contribution to community structure and function (Peñuelas and others 2019). High resource availability and quality are likely to support a higher number of co-occurring species, and higher biodiversity is considered to enhance the ecosystem’s productivity (Cardinale and others 2009). Incorporation of material into faunal biomass together with uptake and recycling rates is essential in directing ecosystem processes such as the storage and turnover of carbon, which will transcend to higher trophic levels and affect biogeochemical cycling on an ecosystem scale (Snelgrove and others 2014). Therefore, shifts in resources that simplify community composition can alter carbon and nutrient recycling towards faster turnover rates and smaller carbon and nutrient pools (Sobral and others 2023). This emphasizes the importance of exploring how biodiversity, stoichiometry and process rates of biological communities are affected by changing resources across environmental scales.
Coastal areas hold a large variety of different habitats and environmental conditions and can sustain high biodiversity and production (Snelgrove and others 2014). However, by being at the interface between land and the open sea, they often receive excess amounts of land-derived organic matter and nutrients (Nixon 1995; Peñuelas and others 2019). Hence, coastal ecosystems play an important role in transforming, retaining, and removing organic matter and nutrients through various biogeochemical processes before reaching the open sea (Asmala and others 2017). Part of the carbon and nutrients entering the systems are retained and recycled in animal biomass (Allgeier and others 2017; Carstensen and others 2020). For example, benthic macrofauna are dominating the living organic carbon pool in many coastal systems. In the Baltic Sea, macrobenthic invertebrates can represent more than 25% of the total sediment organic carbon stock even in vegetated shallow habitats (Scheffold and Hense 2020; Rodil and others 2022). These consumers can modify sediment carbon, nitrogen and phosphorus retention and recycling processes directly through ingestion, biomass production, respiration, and excretion, and indirectly via bioturbation (Josefson and Rasmussen 2000; Vanni 2002; Allgeier and others 2017). As the metabolic processes are species-specific and size-dependent, the benthic community composition will regulate the turnover of benthic carbon and nutrients pools (Atkinson and others 2017; Villnäs and others 2022).
The contribution of benthic fauna to carbon and nutrient retention and recycling shows high variation over spatial scales (Gammal and others 2019; Villnäs and others 2019), because the benthic community composition varies in response to differences in environmental conditions, such as sediment characteristics (Thrush and others 2003) and available food sources (Marcelina and others 2018). While benthic primary production can contribute to fuel benthic consumers in shallow euphotic sediments, strong benthic–pelagic coupling is key for sustaining benthic communities in aphotic areas (Graf 1992). Indeed, as benthic macrofauna in aphotic sediments rely on sinking organic matter as the primary food source, benthic communities are generally food limited (Josefson and Rasmussen 2000; Ehrnsten and others 2019) and show strong responses to variations in pelagic production (Graf 1992). However, as benthic consumers are considered stoichiometrically homeostatic (Persson and others 2010), changes in food sources may create imbalances between consumers and their resources, affecting community structure and function (Atkinson and others 2017).
In coastal areas, terrestrial runoff, sewage discharge and upwelling events often bring high quantities of organic matter and nutrients (Haapala 1994; Savage 2005; Carstensen and others 2020), which may change both the quantity and quality of available food sources for the benthic consumers. While organic matter in dissolved form may be a potential food source for suspension feeders (Kahma and others 2020), many of the benthic species utilize particulate organic matter in the water column and in the sediment. Even though most of the carbon and nutrients from runoff are in dissolved form, some fraction of the organic matter flocculates rapidly in estuaries, forming aggregations, which sink to the sediments (Asmala and others 2014). Terrestrial runoff is likely to increase the quantity but lower the quality of organic matter in the water column due to high C:N:P ratios, but the stoichiometric ratios are strongly dependent on land use of the discharge area (Asmala and others 2013). Moreover, increasing land use in the coastal regions can add inputs of terrestrial silt and clay to coastal habitats. The increased mud content in the sediments often reduces the benthic faunal diversity and abundance (Thrush and others 2003, 2004), altering the structure and function of the benthic communities (Pratt and others 2014).
In contrast, additional nutrients provided by upwelling events in the outer sea could enhance pelagic primary production (Asmala and others 2013, 2017) and increase the availability of autochthonous organic matter for the benthic consumers. Hence, the quality of the organic matter is likely to change from land towards the open sea, with the amount of allochthonous matter decreasing and autochthonous matter increasing along the gradient. This pattern might also be visible between depths, as benthic primary production ceases with light availability in deeper areas. Moreover, spatial differences in the quality of organic matter are also evident in stable isotope signatures. For example, organic matter originating from terrestrial sources can be discerned due to a more depleted signatures of carbon (δ13C; Fry 2006), and microbially processed organic matter, such as treated sewage, can be detected by its elevated δ15N values in comparison with autochthonous sources (Savage 2005).
Benthic macrofauna are important drivers of carbon and nutrient dynamics in marine ecosystems (Vanni 2002; Allgeier and others 2017). However, the carbon and nutrient balance is disrupted in coastal areas due to anthropogenic activities. For instance, in the Baltic Sea, the organic carbon and nutrient loading is increasing as a function of climate change (Meier and others 2023). As multiple stressors continue to affect coastal areas and alter the stoichiometry and balance of available resources, it is essential to study the link between biodiversity, structure, and process rates of benthic faunal communities. In this study we sampled two contrasting benthic habitats (shallow sandy and deep muddy sediments) over a land-to-sea gradient in the Northern Baltic Sea. The study sites closer to land receive inputs of carbon and nutrients from two water treatment plants and from a river, where forest, urban areas, and agriculture dominate the catchment area, but the water quality improves towards the open sea (Asmala and others 2013; Asp and others 2020). We explored how the benthic community composition (biodiversity and biomass), stoichiometric characteristics (C, N and P content and ratios), and community functions (respiration rate and secondary production) vary across the gradient from the inner archipelago towards the open sea in response to environmental drivers. We determined variation in food sources for the benthic consumers (seston and sediment) by using Chlorophyll- a (Chl a) as a proxy for food availability, and Chl a: pheophytin (pheo) together with C:N:P and stable isotopes as indicators of the quality of resources. The dual isotope approach was used to explore how variation in terrestrial loading (δ13C) and nutrient loading (δ15N) was reflected in the benthic consumers. We hypothesize that the innermost sites would have low-quality food for the benthic consumers, which might reduce the macrofaunal biodiversity, biomass, C, N, and P stocks, respiration rates and secondary production compared to outer sites. In contrast, we predict that the outer sites with connection to the open sea could provide more autochthonous food for the benthos and support higher faunal biodiversity, biomass, C, N, and P stocks, and community process rates, enhancing overall ecosystem functioning. In addition, we expected that differences in the benthic community composition or in the food quality would be reflected in the C:N:P ratios of the macrofaunal communities along the gradient.
Materials and Methods
Study Area
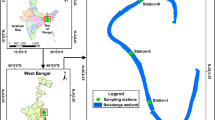
We sampled two contrasting habitats (shallow sandy and deep muddy sediments) across a land-to-sea gradient in Ekenäs archipelago, western Gulf of Finland, in August 2020 (Figure 1). We sampled nine deep aphotic muddy sediments (15–30 m) and seven shallow euphotic sandy sites (3–8 m) along the gradient. The innermost sampling sites were located right outside the city of Ekenäs, south from the Karjaanjoki river mouth and Pojo Bay estuary. In contrast, the outermost sites were in the exposed outer archipelago, with a direct connection to the open sea and characterized by highly variable environmental conditions due to regular upwelling events (Haapala 1994, Figure S1).
Environmental Parameters
Water depth, temperature (°C), salinity, and bottom water oxygen concentration (mg/L, %) were measured with CTD and YSI proODO sondes. Water samples were collected from the surface and bottom water (1 m above the sea floor) with a 2 L Limnos and analysed for total nitrogen and phosphorus concentrations, respectively (Koistinen and others 2017, 2019), and for dissolved NH4+–N, (NO3− + NO2−)–N, NO2–N, PO43−–P and Si (Koroleff 1976). Water nutrients were measured using photometric analyser, except ammonium, which was determined manually using a spectrophotometer. To estimate the fresh pelagic food source available for the benthic fauna, we measured chlorophyll a (Chl a) and pheophytin (pheo) concentrations in the surface water; 100 mL of water was filtered (Whatman GF/F, Ø 25 mm), and Chl a concentration was analysed using fluorescence spectrophotometer after 95% ethanol extraction for 24 h (Lorenzen 1967). In addition, subsequent acid addition enabled the determination of the pheophytin concentration (Lorenzen 1967). Another set of filters were dried for 24 h at 60 °C, capsulated in tinfoil and analysed for seston C and N content, and stable isotopes δ13C and δ15N using an elemental analyser interfaced to an isotope-ratio mass spectrometer at UC Davis. Stable isotopes are expressed using δ notation, as parts per thousand (‰) difference from the international standards Vienna Pee Dee belemnite (for C) and air (for N). Seston P content was analysed as particulate organic phosphorus according to Solórzano and Sharp (1980).
Two replicate sediment samples per site were obtained with a Gemax twin corer (63.6 cm2) at the muddy sites and with a Haps corer (145 cm2) at the sandy sites. We analysed the surface sediment layer (0–1 cm) for organic matter content (OM %), C, N, and P (%) content, stable isotopes δ13C and δ15N, Chl a and pheo. Sediment OM was measured as a loss of ignition (LOI %) by combusting the sediment for 3 h at 500 °C. The sediments used for pigment and elemental analyses were freeze-dried for 72 h in − 70 °C and homogenized. To measure the sediment Chl a content we extracted the sediment for 24 h with 90% acetone and measured the extraction spectrophotometrically. Acidification was included to identify phaeopigments from Chl a (Lorenzen 1967). Sediment organic C and N content together with stable isotopes δ13C and δ15N was analysed using an elemental analyser connected to a continuous flow stable isotope-ratio mass spectrometer at University of Jyväskylä. As negligible contents of inorganic C (Schumacher 2002) and N (Jilbert and others 2011) are reported for the Baltic Sea sediments, the organic fraction can be used to represent the total sediment C and N content. Sediment total P (Ptot) content was determined as particulate phosphorus after Solorzano and Sharp (1980). The sediment organic P fraction (Porg) was analysed after the Williams protocol by first combusting the sediment and extracting it in 1 M HCl according to Ruban and others (1999).
Benthic Macrofauna Community
We sampled benthic macrofauna (3 replicates) with a box corer (400 cm2) at the muddy sites, while a Haps corer (145 cm2) was used at the sandy sites. We hand-picked the animals for elemental analyses from the 0.5 mm sieve into filtered seawater, and the rest of the sample was preserved in 70% ethanol. Fauna selected for elemental analyses were frozen, bivalves were dissected to separate the shells and tissue, and all animal tissue were freeze-dried, homogenized, and encapsulated in tin cups. The C and N content together with stable isotopes (δ13C and δ15N) was determined as described for the sediment. Faunal P content was analysed using the molybdate-blue reaction (Koistinen and others 2019) and measured with a photometric analyser. We selected similar-sized individuals to minimize the variation in species C, N, and P content (Mäkelin and Villnäs 2022), and for the clam M. balthica three size classes (5 mm, 10 mm, and 15 mm) were analysed. We determined the elemental content for all species that had sufficient biomass for the analysis from each sampling site (3–5 replicates per species). Additionally, the benthic fauna were analysed for species richness (to the lowest taxonomic level possible), abundance (individuals m−2), and biomass (dwt g m−2).
Data Analysis
Environmental Parameters
To describe the land-to-sea gradient, we calculated the distance (km) of each sampling site based on its geographic position, from the innermost site outwards (Figure 1). Prior to running analyses, we explored interrelations between all measured environmental variables with draftsman’s plots and Pearson’s correlations. Log10 transformation was applied for seston and sediment C:N:P and Chl a:pheo to make the data more robust for estimating relative changes (Isles 2020). All elemental ratios are presented as molar ratios. To identify which factors were driving the environmental differences across the land-to-sea gradient, we ran a principal component analysis (PCA, PRIMER 7). To test if the environmental conditions differed statistically across the land-to-sea gradient we used linear regressions run in SPSS (IBM SPSS Statistics, version 27.0).
Benthic Macrofauna Community
The benthic community diversity was calculated as number of species. The community biomass was calculated by multiplying the average individual biomass of each species with the corresponding abundance. The biomass was calculated as total biomass (including bivalve and gastropod shells) and as tissue biomass (excluding shells). To estimate the biomass of the M. balthica population accurately we divided the population into five size classes (juveniles < 1 mm, 2–7 mm, 8–12 mm, 13–17 mm, and 18–23 mm) and calculated the biomass separately for each size. The Marenzelleria spp. population was also divided into size classes (small ≤ 0.9 mm, medium ~ 1 mm, and large ≥ 1.1 mm) after the width of the 5th segment. M. balthica biomass dominance was calculated as % from the total community tissue biomass. We used multidimensional scaling plots (MDS) in PRIMER 7 to explore how the benthic community abundance and biomass varied between the two habitats and over the land-to-sea gradient.
The faunal C, N and P stocks (g m−2) were calculated by multiplying the tissue dry weight of each species by the analysed C, N and P %. As the elemental content was measured only for three sizes of M. balthica, we used the content of 5 mm bivalves to represent sizes between 0 and 7 mm, 10 mm for 8–12 mm, and 15 mm for 13–23 mm. We used elemental content values from the literature for species we were unable to measure due to low biomass (Liess and Hillebrand 2005; Brey and others 2010). The total community C, N, and P content was calculated as a sum of the elemental content of each species. The C:N:P ratios of the benthic communities were calculated based on the total organic community C, N, and P content (g m−2).
Benthic community tissue biomass was estimated into ash free dry weight (AFDW) by using conversion factors (Rumohr and others 1987, Brey and others 2010). The benthic community respiration rates and secondary production were determined from AFDW by assuming 50% of the AFDW to be carbon (Wijsman and others 1999; Rodil and others 2020b). Respiration was calculated by using the Mahault and others (1995) formula for shallow water benthos R = 0.0174W0.844, where R is the respiration (C mg m−2 d−1), and W is the average individual biomass as C mg. The daily individual respiration rates were multiplied by the corresponding total abundance of each species. Community respiration rates were calculated by summing the respiration of each species. The formula is valid for temperature between 15 and 20 °C, and to correct the respiration rates for the lower temperatures we assumed a Q10 = 2 (Mahault and others 1995). Secondary production was calculated after Edgar (1990) as P = 0.0049B0.80T0.89, where P is the individual production (C µg day−1), B is the biomass (µg AFDW), and T is the water temperature between 5 and 30 °C, thus no corrections for temperature were required. The average individual production rates were multiplied by the total abundance of each species, and the community production was calculated as a sum of the production rates of each species. The production to biomass ratio (P:B) was calculated by dividing the community secondary production with the community carbon stock.
Linear regressions were used to test if the benthic community composition, stoichiometry, stable isotopes, and process rates differed across the land-to-sea gradient. The relationship between the independent variable distance and the dependent variable was tested in SPSS and run separately for shallow sandy and deep muddy sites. The sites showed some heterogeneity of variance in faunal biomass, C, N and P content caused by a larger number of observations at the outer end of the gradient compared to inner sites as indicated by significant differences in Levene’s test (p < 0.05). However, this heteroskedasticity was an outcome of our sampling strategy rather than the variation in measurements, and the standard residuals (± 3) showed no outliers. In addition, linear regressions were used to explore the relationship between M. balthica dominance and community functions. Pearson correlations were used to test if the community C:N:P ratios were comparable those of the bivalve M. balthica. One-way PERMANOVA (PRIMER 7) was used to test if the P:B differed between the two habitat types.
To analyse the stable isotope patterns of the benthic fauna, we selected M. balthica and Marenzelleria spp. because these two species were the most dominant in abundance and biomass, and both were present across the land-to-sea gradient at muddy and sandy sites. Linear regressions were used to test whether the δ13C and δ15N signatures of these two taxa differed across the gradient, and if the patterns observed in the faunal stable isotope values followed the isotope signatures of seston and sediment. Finally, we used distance-based linear models (DistLM in PERMANOVA + for PRIMER 7, Anderson and others 2008) to study the contribution of biological (species richness, M. balthica biomass dominance) and environmental factors to benthic community C stocks and process rates. We pre-selected environmental parameters for the analysis because they showed strong collinearity (rp > 0.8, Table S1). In addition, we removed temperature and salinity from predictors, because they showed little variation over the land-to-sea gradient. The DistLM was run with different subsets of predictor variables using both stepwise and forward selection procedures. When both procedures agreed, we chose the best models based on lowest AICc values and highest explanatory rate R2.
Results
Environmental Parameters
The first two axes of the PCA explained 60% of the environmental variation in the study area (Figure 2). Axis PC1 explained 35% of the total variation and described the differences between deep muddy and shallow sandy sites, as it was regulated by depth, oxygen conditions, and sediment characteristics. The water column was stratified at the muddy sites (thermo- and halocline); thus, they had lower temperature, lower oxygen conditions and higher salinity compared to sandy sites (Figure 2 and S1, Tables S2 and S3). The muddy sites had higher organic matter content, but lower Chl a and sediment δ15N compared to sandy sediments (Figure 2, Tables S1 and S2). The carbon and nutrient rich surface sediment layer (0–1 cm; without fauna) contained on average 92 ± 45 g C m−2, 11 ± 7 g N m−2, and 0.6 ± 0.2 g Porg m−2 at muddy sites, and 94 ± 29 g C m−2, 13 ± 3 g N m−2, and 5 ± 2 g Porg m−2 at sandy sites.
The PC 2 axis described the environmental variation along the gradient from the inner to outer archipelago (26% of total variation; Figure 2). PC2 was characterized by distance, seston δ13C, δ15N, Chl a, Chl a: pheo, and sediment δ13C and C:N (Figure 2). Although the environmental conditions between the two habitat types differed markedly (PC 1), the environmental variation over the land-to-sea gradient was consistent for both habitat types (PC 2). In muddy sites, distance showed a significant relationship with increasing seston and sediment Chl a: pheo and δ13C, as well as with decreasing seston δ15N (Figure 2, Table S3). In sandy sites, seston and sediment Chl a and δ13C increased with distance, whereas seston δ15N decreased. In addition, oxygen conditions increased, and bottom water nitrogen oxides decreased across the gradient (Table S3). The increasing seston and sediment Chl a towards the open sea indicates an increase in the quantity of food for the benthic consumers at sandy sites. Moreover, the increasing seston Chl a:pheo together with increasing δ13C and decreasing δ15N values suggests that the quality of food improves across the land-to-sea gradient in both habitat types (Figure 3A, Tables S2and S3). Although the change in Chl a, Chl a:pheo, and carbon and nitrogen stable isotope values indicates a difference in food sources for the benthic consumer at the study area, we did not observe significant trends in seston or sediment C:N:P ratios across the gradient (Table S3).
A Variation in stable isotopes δ13C and δ15N of seston and sediment, indicators of benthic food sources, across the land-to-sea gradient. Outermost sandy site (sand 16, circled) marked as an outlier. B The δ13C and δ15N of the bivalve M. balthica and the polychaete genus Marenzelleria spp. changed over the land-to-sea gradient in response to variation in food sources. Statistically significant relationships are illustrated with a trendline, while regressions are presented in Tables S3, S4 and S5.
Benthic Community Composition
The two soft-sediment habitats along the gradient were dominated by typical Baltic Sea taxa, such as the tellinid bivalve Macoma balthica (Linnaeus, 1758), the invasive polychaete genus Marenzelleria spp. (Mesnil, 1896), the amphipod Monoporeia affinis (Lindström, 1855), the priapulid Halicryptus spinulosus (von Siebold, 1849), and the isopod Saduria entomon (Linnaeus, 1758). In addition, the ragworm Hediste diversicolor (Müller, 1776), the gastropods Hydrobiidae (Stimpson, 1865), and the clams Cerastoderma glaucum (Poiret, 1789) and Mya arenaria (Linnaeus, 1758) were common at the sandy sites.
The benthic community biomass increased over the land-to-sea gradient in both habitat types, with an average tissue dry weight of 89 ± 46 g m−2 at muddy sites and 166 ± 98 g m−2 at sandy sites (Figure 4, Table 1 and S4). However, species richness differed significantly only in sandy sediments, where the number of species increased significantly towards the open sea (Table S4). The highest number of species, 12 ± 1 spp., was found at the outermost sandy site (sand 16, Figure 4, Table 1). At muddy sites, the communities were most diverse in the middle of the gradient (mud 6) with an average of 10 ± 1 spp., but no statistically significant trends were observed (Figure 4, Table 1 and S4).
A The number of species increased across the land-to-sea gradient at sandy sites. B The tissue biomass of the benthic communities increased towards the open sea in both habitat types and the clam M. balthica dominated the communities especially in muddy sites. C The biomass dominance of M. balthica decreased towards the open sea. Statistically significant relationships are illustrated with a trendline, while regressions are presented in Table S4.
The same species were driving the differences in benthic community abundance and biomass (MDS plots Figure S2). The main differences in the benthic community structure across the land-to-sea gradient were explained by the abundance and biomass increase of M. balthica towards the outer sites (Figure S2). The Baltic clam M. balthica dominated the benthic communities by forming on average 74% ± 20% of the total tissue biomass at muddy sites and 46 ± 26% at the sandy sites (Figure 4). However, even if the biomass and abundance of M. balthica increased across the land-to-sea gradient, their biomass dominance decreased (Figure 4). This implies that the contribution of other species for community biomass increased towards the open sea. Indeed, high densities of the amphipods Monoporeia affinis were noted at the outer muddy sites and Corophium volutator at the outer sandy sites, whereas Chironomidae were only found in muddy sediments in the inner archipelago (Figure S2). The differences between the two habitat types were explained by species such as the bivalves M. arenaria and C. glaucum, the polychaetes H. diversicolor and Pygospio elegans, and the gastropods Hydrobiidae, which were only found at the sandy sites (Figure S2).
Benthic Community Stoichiometry and Stable Isotopes
Benthic faunal C, N and P stocks followed the same trends as shown in community biomass. In both habitat types, the elemental content of the benthic communities increased across the land-to-sea gradient (Figure 5, Table S4). Muddy sediment communities contained between 1.0–10.4 g C m−2, 0.2–1.9 g N m−2, and 0.02–0.17 g P m−2, whereas sandy sediment communities between 4.4–19.6 g C m−2, 0.9–3.8 g N m−2, and 0.1–0.4 g P m−2 (Figure 5, Table 1). When comparing to the organic C, N and P content of the sediment surface (uppermost 1 cm), the benthic fauna stored, on average 6% C, 9% N, and 18% P in muddy sediments, and 14% C, 20% N and 70% P in the sandy sediments. Despite the large variation in the faunal carbon and nutrient stocks, we found no differences in the benthic community C:N:P ratios across the gradient (Table S4). The only exception was community C:N, which differed across the land-to-sea gradient at muddy sites. The community stoichiometry followed the C:N:P ratios of the most dominant species, M. balthica (Table S5).
A The benthic community carbon stocks B respiration rate and C secondary production increased across the land-to-sea gradient. Community nitrogen and phosphorus stocks followed the same trend as carbon (not shown). D Community production to biomass ratio (P:B) differed between the two habitat types. E M. balthica biomass dominance showed a negative relationship with benthic community functions, such as the C stock, indicating that increasing contribution of other species to community biomass enhanced community stocks and process rates. Statistically significant relationships are illustrated with a trendline, while regressions are presented in Tables S4 and S5.
The benthic fauna distinctly responded to variation in food sources as the δ13C and δ15N of the bivalve M. balthica and the polychaete genus Marenzelleria spp. showed significant change across the land-to-sea gradient (Figure 3B, Table S4). In both taxa, the δ13C values were more negative at the innermost sites and increased towards the open sea (Figure 3B, Table S4). The faunal δ13C showed strong correlation with seston and sediment stable isotope values in both sandy and muddy habitats (Figure 3B, Table S5). The δ15N of Marenzelleria spp. decreased towards the open sea in both habitat types, following the trends observed in seston δ15N (Figure 3B). However, the δ15N of M. balthica decreased in sandy habitats but increased in muddy habitats across the land-to-se gradient. The δ15N of M. balthica correlated with seston and sediment δ15N in both habitat types. The δ15N of Marenzelleria spp. showed positive correlation with seston δ15N on sandy sites, but a negative correlation with sediment δ15N on muddy sites (Table S5).
Benthic Community Process Rates
In both habitat types the benthic community respiration and production rates increased across the land-to-sea gradient (Figure 4, Table 1 and S4). At the muddy sites the benthic community respiration rates varied between 7 and 144 mg C m−2 d−1, and between 15 and 232 mg C m−2 d−1 at the sandy sites (Figure 5, Table 1). The secondary production ranged between 6 and 103 mg C m−2 d−1 at the muddy sites and between 13 and 210 mg C m−2 d−1 at the sandy sites (Figure 5, Table 1). The production to biomass ratio was higher at the muddy sites than sandy sites (F1,45 = 13.284, p < 0.001, Figure 5). The P:B showed a slight decrease across the land-to-sea gradient in muddy sites, but no significant trend was observed in sandy sites, although in both habitat types there was high variation between the replicate samples (Figure 5, Table S4).
According to the DistLM analyses, at muddy sites sediment Chl a:pheo and M. balthica dominance explained 63% of the variation in benthic community carbon stocks. In turn, sediment Chl a:pheo together with species richness and bottom water N:P were the most important factors explaining 77% of the variation in benthic community respiration and 76% in secondary production at muddy sites (Table 2). Sediment Chl a:pheo showed strong collinearity with seston Chl a:pheo, δ13C and δ15N highlighting the importance of food quality for the faunal carbon stocks and process rates (Table S1). At sandy sites, M. balthica biomass dominance, species richness, seston N:P and sediment Chl a were the most important factors predicting benthic community stocks and process rates (Table 2). The models explained 89% of the variation in the faunal carbon stocks, 92% of respiration rate, and 91% of secondary production (Table 2). Benthic community carbon stocks, respiration and secondary production increased with decreasing M. balthica biomass dominance, indicating that the contribution of other species to community biomass enhances community stocks and process rates at sandy sites (Figure. 5, Table S4).
Discussion
Our study shows that increasing resource quality across the land-to-sea gradient modifies benthic macrofaunal diversity and community composition, which in turn enhances important ecosystem functions provided by the benthic consumers, such as carbon and nutrient storage, respiration rates, and secondary production. While food quality explained a major part of the variability in faunal stocks and process rates in muddy habitats, a higher species richness together with decreasing biomass dominance of the key species M. balthica were the major factors influencing increasing faunal community stocks and process rates at the sandy sites.
Environmental Parameters
Our data show that the quality of resources for the benthic consumers differs between the two habitat types and increases from inner archipelago towards the open sea. In general, the available primary resources for the benthic consumers in bare sediment habitats of the coastal Baltic Sea are phytoplankton, benthic microalgae, and detritus from autochthonous or allochthonous sources (Marcelina and others 2018). In aphotic soft-sediments, detritus is the basal food source for the benthic consumers (Graf 1992), and the sinking time of the organic matter can vary between 15 and 30 days (30 m depth; Rodil and others 2020a). In contrast, at the shallow sandy sites, the sinking time is short leading to a close benthic–pelagic coupling. Moreover, in the shallow areas, light reaches the seafloor and benthic primary production occurs, which enhances food quality for the benthic consumers (Marcelina and others 2018). In our study, this was indicated by the higher sediment Chl a at sandy sites and by faunal δ13C signals, which correlated strongly with the δ13C of the shallow sandy sediments. In addition, organic matter derived from drifting macroalgae can form a large source of energy and nutrients for the macrofauna in shallow areas (Kahma and others 2020). Thus, the sandy sediments likely have higher availability of fresh food sources and a potential to support higher consumer diversity and biomass compared to deep aphotic sediments.
Even though we did not find significant differences in the water nutrient concentrations or in seston and sediment C:N:P at the time of sampling, previous studies indicate that there is a strong gradient in background nutrient loading in the study area (Asmala and others 2013; Asp and others 2020). Our measurements emphasizes the importance of regular water quality monitoring in the study area, which gives us a good understanding of the more permanent conditions, even though we could not detect the trend during our sampling. The inner parts of the Ekenäs archipelago are eutrophied and classified as poor based on water quality, whereas the ecological status improves to moderate towards the open sea (Asp and others 2020). The inner archipelago receives inputs of carbon and nutrients from Karjaanjoki river, where forest, urban areas, and agriculture dominate the catchment area (Asmala and others 2013, 2014; Asp and others 2020). This was supported by the more negative seston and sediment δ13C values at the innermost sites indicating terrestrial runoff (Fry 2006; Kahma and others 2020). In addition, two water treatment plants in the river estuary increase the nutrient load to the inner archipelago (Asp and others 2020), as indicated by the high seston δ15N values close to land suggesting a high contribution of microbially processed organic matter (Savage 2005; Karlson and others 2015a). The dissolved organic matter flocculates and sinks fast at the river estuary; therefore, the material rarely reaches the middle or outer areas of Storfjärden Bay (Asmala and others 2014; Kahma and others 2020). Benthic consumers can directly utilize particulate allochthonous organic matter, but despite a high availability of land-derived organic matter in the sediments they tend to select autochthonous resources (Antonio and others 2010; Bartels and others 2018). Typically, higher consumption of allochthonous material occurs in river mouths and decreases with distance (Antonio and others 2010; Bartels and others 2018).
In contrast, at the outer end of our gradient the primary production is boosted by regular upwelling events that bring nitrate and phosphate from deeper water layers to the surface (Haapala 1994). Seston Chl a and Chl a:pheo together with sediment Chl a:pheo ratio increased along the land-to-sea gradient, showing increased pelagic phytoplankton production at the outer sites. This was supported by the stable isotope signatures, as seston and sediment δ13C were less negative towards the outer archipelago. The lower δ15N values towards the open sea could also indicate nitrogen fixation by cyanobacteria (Karlson and others 2015b). As the Gulf of Finland is generally nitrogen limited, the nitrogen fixation by cyanobacteria boosts pelagic production and supports benthic food webs (Karlson and others 2015b).
Benthic Community Composition, Stoichiometry, and Stable Isotopes
The observed increase in community abundance and biomass across the land-to-sea gradient was mostly caused by an increased occurrence of the key species M. balthica. Interestingly, the biomass dominance of the clam decreased over the gradient and the contribution of other species to community biomass increased. Especially at the sandy sites, the high resource availability and quality likely resulted in an increased number of species across the gradient (compare Cardinale and others 2009). In contrast, at muddy sites the species richness was more affected by sediment characteristics, as the number of macrofaunal species tend to decrease with increasing mud content in the sediments (Thrush and others 2003).
The structural and functional composition of benthic macrofaunal communities varies across spatial gradients (Villnäs and others 2019), but the implications of how such shifts alter the benthic community stoichiometry remain largely unresolved (Cross and others 2003; Atkinson and others 2017). Benthic communities are often stoichiometrically heterogeneous depending on the community composition, as different species and different life stages have different elemental content and ratios. For instance, the chitinous exoskeletons of crustaceans contain mainly carbon but very little nutrients (Elser and others 1996), or animals with high growth rates tend to have higher body P content due to the increased demand of P during rapid growth (Elser and others 2003). Moreover, mismatches between consumers and their food sources alter their stoichiometric characteristics (Bracken 2017). Therefore, we expected that benthic community C:N:P ratios would vary over the land-to-sea gradient but learned that the environmental and the elemental ratios of the benthic communities remained mostly stable. Only in muddy sites the community C:N increased towards the open sea. The community stoichiometry followed the elemental content of the most dominant species, the clam M. balthica, whose N content (%) decreased slightly across the gradient. In addition, the communities contained more crustaceans with high C:N ratio towards the outer sites.
Even though the faunal C:N:P ratios did not vary across the gradient, faunal stable isotope signatures showed clear response to variation in food quality. The clam M. balthica and the polychaete Marenzelleria spp. were the two species present throughout the land-to-sea gradient, and commonly dominating the benthic communities throughout the Baltic Sea (Gogina and others 2016). Both species are facultative suspension and deposit feeders (Dauer and others 1981; Ólafsson 1986). The δ13C of M. balthica and Marenzelleria spp. increased across the land-to-sea gradient in both habitat types, following the variation in seston and sediment δ13C. The δ15N of Marenzelleria spp. decreased across the land-to-sea gradient in both habitat types, but for M. balthica the trends differed between the habitats. M. balthica can switch between suspension feeding and deposit feeding (Ólafsson 1986), and our results indicate that it most likely prefers filter-feeding in the nutrient poor sandy sediments as its δ15N signatures follow the seston. In the muddy habitats the high availability of detritus probably sustains deposit feeding of M. balthica, as its δ15N are closer to sediment (compare Karlson and others 2015a). Also, it seems that Marenzelleria spp. is selecting seston at sandy sites, but sediment at muddy sites, as indicated by its δ15N values. As these species show plasticity in their biological traits, their impact on ecosystem functions is likely to differ between habitats (Törnroos and others 2015).
Benthic Community Stocks and Process Rates
Benthic macrofauna have a significant role in coastal carbon recycling processes, as they retain and recycle carbon and nutrients through their metabolism (Vanni 2002; Allgeier and others 2017). Earlier studies have shown that in the northern Baltic Sea benthic fauna can contribute more than 25% to the total organic carbon pool, and even more than 40% to seafloor respiration (Scheffold and Hence 2020; Rodil and others 2020b, 2022). Our measured C stock, respiration rate and secondary production are in line with other studies conducted in the same area, but lower compared to vegetated sediments or blue mussel beds (Rodil and others 2020b, 2022). At sandy sites with the highest benthic diversity and biomass, faunal tissue contained more organic P than the sediment surface. Importantly, the faunal C, N, and P stocks were relatively stable as large and long-living bivalves dominated the community biomass.
At the muddy sites the benthic community respiration rates varied between 7 and 144 mg C m−2 d−1, and between 15 and 232 mg C m−2 d−1 at the sandy sites. Compared to total seafloor respiration rates measured during the same season in the study area (approximately 70 mg C m−2 d−1 in aphotic muddy and 39 mg C m−2 d−1 in bare sandy sediments; Attard and others 2019), our macrofaunal community respiration contributed between 10 and 200% to total seafloor respiration in aphotic muddy sites and between 30 and 50% in shallow sandy sites. Hence, the benthic macrofauna can function as hotspots of carbon and nutrients emphasizing their importance for overall nutrient uptake and turnover.
At deep muddy sites, food quality was the most important factor increasing the benthic community carbon stocks and respiration and production rates, processes essential for carbon turnover. At shallow sandy sites with close benthic–pelagic coupling, the biological factors such as the decrease in the biomass dominance of M. balthica and increasing species richness explained a significant proportion of the community carbon stocks and process rates. One explanation for the observed trends is that the better food quality towards the open sea sustains high benthic community biomass, which in turn directs high carbon stocks and process rates (Cardinale and others 2009). On the other hand, the increase in the number of species together with reduced M. balthica biomass dominance could lead to higher trait distribution and to better resource partitioning between the species (Karlson et al. 2010), which, in our case, enhanced the carbon and nutrient retention capacity and measured process rates of the benthic community.
We found higher benthic community P:B rates at muddy sites compared to sandy sites, indicating that the muddy sediment communities have faster growth rate, smaller body size, shorter lifespan, and consequently faster turnover of carbon (Wetzel 2001). Similar succession was also observed in muddy sites across the land-to-sea gradient, suggesting that at the outer sites the diverse benthic communities are dominated by long-living large individuals, which form a stable C, N and P stock and have higher contribution to the overall carbon and nutrient retention and removal capacity in the sediments (Villnäs and others 2019; Carstensen and others 2020). Villnäs and others (2019) found similar spatial shifts in macrofaunal trait composition in several Baltic coastal areas, indicating that benthic communities affected by eutrophication consist of smaller fast-growing individuals with traits that enhance the nutrient turnover, but decrease the carbon and nutrient retention capacity of the community. Environmental change caused by intensive land use, eutrophication and global warming could shift the benthic community trait distribution towards faster fluxes and smaller C, N and P pools, which would critically alter the carbon and nutrient retention and recycling capacity of the coastal habitats (Villnäs and others 2019; Carstensen and others 2020; Sobral and others 2023). The results of this study highlight the importance of sustaining diverse benthic macrofaunal communities for maintaining the secondary productivity and carbon cycling in coastal ecosystems.
References
Allgeier JE, Burkepile DE, Layman CA. 2017. Animal pee in the sea: consumer-mediated nutrient dynamics in the world’s oceans. Global Change Biology 23:2166–2178.
Anderson MJ, Gorley RN, and Clarke KR Permanova+ for Primer: Guide to software and statistical methods, PERIMER-e.
Antonio ES, Kasai A, Ueno M, Won N, Ishihi Y, Yokoyama H, Yamasita Y. 2010. Spatial variation in organic matter utilization by benthic communities from Yura river-estuary to offshore of Tongo Sea, Japan. Estuarine Coastal and Shelf Science 86:107–117.
Asmala E, Autio R, Kaartokallio H, Pitkänen L, Stedmon CA, Thomas DN. 2013. Bioavailability of riverine dissolved organic matter in three Baltic Sea estuaries and the effect of catchment land use. Biogeochemistry 10:6969–6986.
Asmala E, Bowers DG, Autio R, Kaartokallio H, Thomas DN. 2014. Qualitative changes of riverine dissolved organic matter at low salinities due to flocculation. Journal of Geophysical Research: Biogeosciences 119:1919–1933.
Asmala E, Carstensen J, Conley DJ, Slomp CP, Stadmark J, Voss M. 2017. Efficiency of the coastal filter: nitrogen and phosphorus removal in the Baltic Sea. Limnology and Oceanography 62:222–238.
Asp T, Holmberg R, Lehmijoki A, and Valtonen M Lohjanjärven sekä Mustionjoen, Pohjanpitäjänlahden ja Tammisaaren merialueen yhteistarkkailujen yhteenveto vuodelta 2019, Länsi-Uudenmaan vesi ja ympäristö ry Julkaisu, (2020) p 14.
Atkinson CL, Capps KA, Rugenski AT, Vanni MJ. 2017. Consumer-driven nutrient dynamics in freshwater ecosystems: from individuals to ecosystems. Biological Reviews 92:2003–2023.
Attard KM, Rodil IF, Glud RN, Berg P, Norkko J, Norkko A. 2019. Seasonal ecosystem metabolism across shallow benthic habitats measured by aquatic eddy covariance. Limnology and Oceanography Letters 4:79–86.
Bartels P, Ask J, Andersson A, Karlsson J, Giesler R. 2018. Allochthonous organic matter supports benthic but not pelagic food webs in shallow coastal ecosystems. Ecosystems 21:1459–1470.
Bracken MES. 2017. Stoichiometric mismatch between consumers and resources mediates the growth of rocky intertidal suspension feeders. Frontiers in Microbiology 8:1297.
Brey T, Müller-Wiegmann C, Zittier ZMC, Hagen W. 2010. Body composition in aquatic organisms – A global data bank of relationships between mass, elemental composition and energy content. Journal of Sea Research 64:334–340.
Cardinale BJ, Hillebrand H, Harpole WS, Gross K, Ptacnik R. 2009. Separating the influence of resource availability from resource imbalance on productivity–diversity relationships. Ecology Letters 12:475–487.
Carstensen J, Conley DJ, Almroth-Rosell E, Asmala E, Bonsdorff E, Fleming-Lehtinen V, Gustafson BG, Gustafsson C, Heiskanen A-S, Janas U, Norkko A, Slomp C, Villnäs A, Voss M, Zilius M. 2020. Factors regulating the coastal nutrient filter in the Baltic Sea. Ambio 49:1194–1210.
Cross WF, Benstead JP, Rosemond AD, Wallace JB. 2003. Consumer-resource stoichiometry in detritus-based streams. Ecology Letters 6:721–732.
Dauer DM, Maybury CA, Erwing RM. 1981. Feeding behaviour and general ecology of several spionid polychaetes from the Chesapeake Bay. Journal of Experimental Marine Biology and Ecology 54:21–38.
Edgar GJ. 1990. The use of the size structure of benthic macrofaunal communities to estimate faunal biomass. Journal of Experimental Marine Biology and Ecology 137:195–214.
Ehrnsten E, Norkko A, Timmermann K, Gustafsson BG. 2019. Benthic-pelagic coupling in coastal seas – modelling macrofaunal biomass and carbon processing in response to organic matter supply. Journal of Marine Systems 196:36–47.
Elser JJ, Dobberfuhr DR, MacKay NA, Schamnel JH. 1996. Organism size, life history. and N: P stoichiometry. Bioscience 46:674–684.
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW. 2003. Growth rate-stoichiometry couplings in diverse biota. Ecology Letters 6:936–943.
Fry B. 2006. Stable isotope ecology. Springer.
Gammal J, Järnström M, Bernard G, Norkko J, Norkko A. 2019. Environmental context mediates biodiversity – Ecosystem functioning relationship in coastal soft-sediment habitats. Ecosystems 22:137–151.
Gogina M, Nygård H, Blomqvist M, Daunys D, Josefson AB, Kotta J, Maximov A, Warzocha J, Yermakov V, Gräwe U, Zettler M. 2016. The Baltic Sea scale inventory of benthic faunal communities. ICES Journal of Marine Science 73:1196–1213.
Graf G. 1992. Benthic-pelagic coupling: a benthic view. Oceanography and Marine Biology Annual Review 30:149–190.
Haapala J. 1994. Upwelling and its influence on nutrient concentration in the coastal area of the Hanko Peninsula, entrance of the Gulf of Finland. Estuarine, Coastal and Shelf Science 38:507–521.
Isles PDF. 2020. The misuse of ratios in ecological stoichiometry. Ecology 101:e03153.
Jilbert T, Slomp CP, Gustafson BG, Boer W. 2011. Beyond the Fe-P-redox connection: preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles. Biogeosciences 8:1699–1720.
Jilbert T, Asmala E, Schröder C, Tiihonen R, Myllykangas J-P, Virtasalo JJ, Kotilainen A, Peltola P, Ekholm P, Hietanen S. 2018. Impacts of flocculation on the distribution and diagenesis of iron in boreal estuarine sediments. Biogeosciences 15:1243–1271.
Josefson AB, Rasmussen B. 2000. Nutrient retention by benthic macrofaunal biomass of Danish estuaries: Importance of nutrient load and residence time. Estuarine, Coastal and Shelf Science 50:205–216.
Kahma TI, Karlson AML, Sun X, Mörth C-M, Humborg C, Norkko A, Rodil IF. 2020. Macroalgae fuels coastal soft-sediment macrofauna: A triple-isotope approach across spatial scales. Marine Environmental Research 162:105163.
Karlson AML, Nascimento FJA, Näslund J, Elmgren R. 2010. Higher diversity of deposit-feeding macrofauna enhances phytodetritus processing. Ecology 91:1414–1423.
Karlson AML, Gorokhova E, Elmgren R. 2015a. Do deposit-feeder compete? Isotopic niche analysis of an invasion in a species-poor system. Scientific Reports 5:9715.
Karlson AML, Duberg J, Motwani NH, Hogfors H, Klawonn I, Ploug H, Svedén JB, Garbaras A, Sundelin B, Hajdu S, Larsson U, Elmgren R, Gorokhova E. 2015b. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio 44:413–426.
Koistinen J, Sjöblom M, and Spilling K in Determining inorganic and organic phosphorus, (ed) Spilling K Biofuels from algae. Methods in molecular biology, 1980 Humana, New York, (2017) pp 87–94.
Koistinen J, Sjöblom M, and Spilling K in Total nitrogen determination by a spectrophotometric method, (ed) Spilling K Biofuels from algae. Methods in molecular biology, 1980 Humana, New York, (2017), pp 81–86.
Koroleff F. 1976. Methods for the chemical analysis of seawater. Meri 7:1–60.
Liess A, Hillebrand H. 2005. Stoichiometric variation in C:N, C:P, and N: P ratios of littoral benthic invertebrates. Journal of the North American Benthological Society 24:256–269.
Lorenzen CJ. 1967. Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnology and Oceanography 12:343–346.
Mahault M-L, Sibuet M, Shirayama Y. 1995. Weight-dependent respiration rates in deep-sea organisms. Deep Sea Research Part i: Oceanographic Research Papers 42:1575–1582.
Mäkelin S, Villnäs A. 2022. Food sources drive temporal variation in elemental stoichiometry of benthic consumers. Limnology and Oceanography 67:784–799.
Marcelina Z, Sokołowski A, Pierre R. 2018. Spatial and temporal variability of organic matter sources and food web structure across benthic habitats in a low diversity system (southern Baltic Sea). Journal of Sea Research 141:47–60.
Meier HEM, Reckermann M, Langner J, Smith B, Didenkulova I. 2023. Overview: The Baltic Earth assessment reports (BEAR). Earth System Dynamics 14:519–531.
Nixon SW. 1995. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–219.
Ólafsson EB. 1986. Density dependence in suspension feeding and deposit-feeding populations of the bivalve Macoma balthica: A field experiment. Journal of Animal Ecology 55:517–526.
Peñuelas J, Janssens IA, Ciais P, Obersteiner M, Sardans J. 2019. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Global Change Biology 26:1962–1985.
Persson J, Fink P, Goto A, Hood JM, Jonas J, Kato S. 2010. To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119:741–751.
Pratt DR, Lohrer AM, Pilditch CA, Thrush SF. 2014. Changes in ecosystem function actoss sedimentary gradients in estuaries. Ecosystems 17:182–194.
Rodil IF, Lucena-Moya P, Tamelander T, Norkko J, Norkko A. 2020a. Seasonal variation in benthic-pelagic coupling: quantifying organic matter inputs to the seafloor and benthic macrofauna using a multi-marker approach. Frontiers in Marine Science 10:3389.
Rodil IF, Attard KM, Norkko J, Glud RN, Norkko A. 2020b. Estimating respiration rates and secondary production of macrobenthic communities across coastal habitats with contrasting structural biodiversity. Ecosystems 23:630–647.
Rodil IF, Lohrer AM, Attard KM, Thrush SF, Norkko A. 2022. Positive contribution of macrofaunal biodiversity to secondary production and seagrass carbon metabolism. Ecology 103:e3648.
Ruban V, López-Sánches JF, Pardo P, Rauret G, Muntau H, Quevauviller P. 1999. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediments. Journal of Environmental Monitoring 1:51–56.
Rumohr H, Brey T, and Ankar S A compilation of biometric conversion factors for benthic invertebrates of the Baltic Sea, Institut für Meereskunde (1987)
Savage C. 2005. Tracing the influence of sewage nitrogen in a coastal ecosystem using stable nitrogen isotopes. Ambio 34:145–150.
Scheffold MIE, Hence I. 2020. Quantifying contemporary organic carbon stocks of the Baltic Sea ecosystem. Frontiers in Marine Science 7:571956.
Schumacher B A, Methods for the determination of total organic carbon (TOC) in soils and sediments, USEPA, (2002) p 1–23.
Snelgrove PVR, Thrush SF, Wall DH, Norkko A. 2014. Real world biodiversity-ecosystem functioning: a seafloor perspective. Trends in Ecology & Evolution 29:398–405.
Sobral M, Schleuning M, Cortizas AM. 2023. Trait diversity shapes the carbon cycle. Trends in Ecology and Evolution. 38:3137.
Solórzano L, Sharp JH. 1980. Determination of total dissolved and particulate phosphorus in natural waters. Limnology and Oceanography 25:754–758.
Törnroos A, Nordström MC, Aarnio K, Bonsdorff E. 2015. Environmental context and trophic trait plasticity in a key species, the tellinid clam Macoma balthica L. Journal of Experimental Marine Biology and Ecology 472:32–40.
Thrush SF, Hewitt JE, Norkko A, Nicholls PE, Funnell GA, Ellis JI. 2003. Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Marine Ecology Progress Series 263:101–112.
Thrush SF, Hewitt JE, Cummings VJ, Ellis JI, Hatton C, Lohrer A, Norkko A. 2004. Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment 2:299–306.
Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology, Evolution, and Systematics. 33:341–370.
Villnäs A, Janas U, Josefson AB, Kendzierska H, Nygård H, Norkko J, Norkko A. 2019. Changes in macrofaunal biological traits across estuarine gradients: implications for the coastal nutrient filter. Marine Ecology Progress Series 622:31–48.
Villnäs A, Mäkelin S, Vanni MJ. 2022. Allometric and stoichiometric traits predict nutrient excretion rates by benthic consumers. Frontiers in Marine Science 9:870308.
Wetzel RG. 2001. Limnology: Lake and river ecosystems. Gulf professional publishing.
Wijsman J, Herman P, Gomoiu M. 1999. Spatial Distribution in Sediment Characteristics and Benthic Activity on the Northwestern Black Sea Shelf. Marine Ecology Progress Series 181:25–39.
Acknowledgments
We want to thank Mikko Kiljunen and Nina Honkanen at the University of Jyväskylä for conducting the elemental and SI analyses. We also thank the staff of UC Davis Stable Isotope facility for seston SI analyses. We thank Laura Kauppi for helping us with Tvärminne monitoring data, Johanna Gammal for GIS expertise, Markku Viitasalo for scientific discussions and Tom Jilbert for advising in sediment analyses and for discussions. We also wish to thank the crew of R/V Augusta, the laboratory staff, and the trainees at TZS for helping in the field and in the lab. This study utilized research infrastructure facilities at Tvärminne Zoological Station, University of Helsinki, as part of FINMARI (Finnish Marine Research Infrastructure consortium). We also thank the Centre for Coastal Ecosystem and Climate Change Research for support.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This study was funded by the University of Helsinki three-year research grant, the Academy of Finland (323212), the Walter and Andrée de Nottbeck Foundation, and the Sophie von Julin Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions: AV, SM, AL, and IR contributed to study conception and design; SM, AV, and IR contributed to data collection; and all authors contributed to analysis and interpretation of results and draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mäkelin, S., Lewandowska, A.M., Rodil, I.F. et al. Linking Resource Quality and Biodiversity to Benthic Ecosystem Functions Across a Land-to-Sea Gradient. Ecosystems 27, 329–345 (2024). https://doi.org/10.1007/s10021-023-00891-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00891-9