Abstract
Carbon (C) sequestrated in the boreal forest ecosystems plays an important role in climate regulation. This study’s objectives were to quantify the differences in the components of the forest C cycle along a 1000 km latitudinal gradient within the boreal region and between dominant coniferous species in Fennoscandia. The study included seven xeric–sub-xeric and eight mesic–herb-rich heath forests dominated by Scots pine and Norway spruce, respectively. The total site carbon stock (CS) ranged from 81 to 260 Mg ha−1. The largest ecosystem component CSs were tree stems, mineral soil, and humus layer, representing 30 ± 2%, 28 ± 2%, and 13 ± 1% of total CS, respectively. On average, the spruce sites had 40% more C than the pine sites, and CS stored in most compartments was higher on spruce than on pine sites. As exceptions, understorey vegetation and litter layer had a larger CS on pine than on spruce sites. The northern sites had an average of 58% less C than the southern sites. Humus layer CS was the only compartment showing no latitudinal trends. Northern sites had a significantly larger fine and small root CS and understorey CS than southern sites. Most CS compartments were significantly correlated with litterfall C transfer components. Dissolved organic carbon (DOC) flux in throughfall was positively correlated with the aboveground tree compartment CS. Our study revealed patterns of C distribution in major boreal forest ecosystems along latitudinal and fertility gradients, which may serve as a reference for Earth system models and in the evaluation of their projections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
We report carbon stocks (CS) and transfers in 15 boreal (60-69 °N) forest ecosystems
-
Total site CSs ranged 81–260 Mg ha-1; stems, soil and humus layer were largest CSs
-
Humus layer CS was the only compartment showing no latitudinal trend
Introduction
Boreal forest ecosystems account for about 32% of global forest ecosystem carbon (C) stocks (Pan and others 2011). The sequestration and change of C pools are driven by environmental factors such as climate, site fertility, and moisture conditions (Ortiz and others 2013; Fernández-Martinez and others 2014; Todd-Brown and others 2013; Ťupek and others 2016; Nanko and others 2017) and are mediated by the structure and species composition of the tree stand and forest vegetation as a whole (Andivia and others 2016; Dawud and others 2016; Shanin and others 2014; Vesterdal and others 2013).
Boreal forests are largely characterised by coniferous trees. In Fennoscandia, the dominant coniferous species are Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.), which, according to National Forest Inventory, represent 50% and 30% of stem volume in Finnish forests, respectively (Korhonen and others 2021). As producers of economically valuable raw material, these species have also been favoured in forest management. Scots pine dominates xeric and sub-xeric upland sites on mineral soil of lower fertility, while Norway spruce thrives on mesic and herb-rich sites (Ilvessalo 1927). Characteristically, a large proportion of C in boreal forests is stored in relatively recalcitrant forms in soil organic matter (SOC), resulting from the short and cool growing seasons and the low substrate quality of the litter, which reduce soil microbial activity (Fierer and others 2005). For long periods, boreal forests have acted as an important carbon sink to balance out the increasing atmospheric CO2 concentration. In addition to the importance of boreal forest C in climate regulation, it holds a multitude of functional roles and is a crucial factor behind forests’ ecosystem services.
In Fennoscandia, the SOC decreases, and the proportion of forest understorey vegetation biomass to that of the tree stand canopy increases, from south to north (Merilä and others 2014; Lehtonen and others 2016). Although the literature on boreal forest C is vast, comprehensive studies that include both the distribution of C in different compartments of the ecosystem and the transfers of C are relatively scarce (Berggren Kleja and others 2008; Hansson and others 2013; Laganière and others 2015; Melvin and others 2015).
In this study, we examined C stocks and transfers (Figure. 1) in 15 boreal forest ecosystems on mineral soil in Finland, based on the measured data from the sites belonging to the UNECE ICP Forests Level II programme (that is, intensive monitoring programme; Lorenz and Fischer 2013). The sites include two site fertility moisture levels dominated by Scots pine (Pinus sylvestris (L.)) and Norway spruce (Picea abies (L.) Karst.), respectively (Salemaa and others 2008). Our objectives were to quantify the differences in the components of the forest C cycle between latitudinal locations within boreal regions (southern vs northern boreal) and between dominant coniferous species. In particular, we aimed to evaluate species-specific and latitudinal patterns of measured C stocks in plant biomass and soil.
Materials and Methods
Sample Sites
The data were collected during 1996–2009 from seven xeric and sub-xeric (hereafter xeric) and eight mesic–herb-rich (hereafter mesic) heath forests dominated by Scots pine (hereafter pine) and Norway spruce (hereafter spruce), respectively. For stand and site characteristics see Table 1 and Merilä and others (2014), and for the composition of forest floor vegetation see Table 2. The study sites were divided in northern (northern or middle boreal) and southern (southern boreal) sites (Ahti and others 1968); northern sites included three pine and three spruce sites, whereas southern sites included four pine and five spruce sites (Figure. 2a).
a, b Location of the study sites (left) and the design of the intensive monitoring plots (ICP Forests Level II) in Finland (right). On each monitoring plot, one subplot is reserved for vegetation monitoring, another one for needle sampling and a third one for monitoring of stand litterfall, throughfall, and percolation water.
The sites are part of the European intensive forest monitoring network (Level II), established under the UNECE ICP Forests monitoring programme (Lorenz and Fischer 2013). The monitoring activities on the study sites started in 1995–1999. Twelve sites are in managed forests. The stands are naturally regenerated, apart from site nr. 23, which was planted for spruce. The major management measure carried out on these study sites is thinnings, the aim of which is to direct the biomass production to best growing stems and thus enhance timber production and prevent self-thinning of the stand. Five of the sites were slightly thinned after the dormant season 2004–2005 when measurements for calculation of stand biomass were carried out: site nr. 16 was thinned in 2005, nrs. 5, 11, and 23 in 2006, and site nr. 6 in 2008. The thinnings may have had a minor effect on the results of litterfall (calculated as mean 1996–2007) and understorey vegetation (sampled in 2009), as their sampling period occurred at least partly after the thinnings. However, no drastic changes were noticeable in these variables in comparison with pre-thinning years (data not shown). Three sites are in protected conservation areas where forest management has not been carried out for at least the last 50 years. Most of the pine sites are on sorted glaciofluvial material, where sand fraction is dominant, and the spruce sites on till soils representing a mixture of different texture classes with sand fraction as the dominant fraction. Each site consists of three subplots (30 × 30 m) and a surrounding mantle (Figure. 2b). The three subplots are reserved for different purposes: one is for destructive sampling (for example, deposition, foliar, litterfall, soil, and soil water sampling), the second one for vegetation observations and the third one for tree growth measurements.
Estimation of Carbon Stocks (CS)
Stand biomass. The diameter at a height of 1.3 m, tree height, and crown length were measured for each tree on all three subplots of each 15 monitoring sites during the autumn of 2004 and spring of 2005, as described in Merilä and others (2014). The biomasses of individual trees were estimated using allometric equations by Repola and others (2007) except for needle biomass, which was calculated using equations of Repola (2009). The biomasses of individual trees were summed to obtain total stand biomass (kg ha−1 dry weight). For further information, see Supplementary Material.
Sampling of stem wood, bark, living, and dead branches for C analysis. To determine the C concentrations in the aboveground tree compartments (stem wood, bark, living, and dead branches), five randomly selected trees from the dominant canopy layer were harvested on each of the sites in 2006 and sampled for C analysis as described in detail in Ukonmaanaho and others (2008), Merilä and others (2014), and in Supplementary Material.
Sampling and pre-treatment of needles for C analysis. Sample branches (all present needle year classes) were collected in late October–early December 2007 with a pruning device from the uppermost third of the living crown of 10 predominant or dominant sample trees on one subplot of each site, as described in Merilä and others (2014) according to Rautio and others (2020). See Supplementary Material for details. In the computation of the needle C pool, we used the mean C concentration of all needle year classes present in ≥ 50% of the sample trees of each site, weighed by their share of biomass in sample branches.
Understorey vegetation and litter (L) layer. The aboveground biomass of understorey vegetation was harvested from 28 squares (30 × 30 cm2 each) located systematically along the sides of the vegetation monitoring subplots of the Level II monitoring sites (Merilä and others 2014) in July–September 2009. For each site, the aggregated area of the squares was 2.52 m2. The living biomass and necromass of understory vegetation were separated by plant species in each sample (Merilä and others 2014). Living vascular plant species biomasses were further separated for leaves and stems and moss and lichen species (or species groups) for upper (living) and lower (dead; considered as L layer) part.
The collection of the litter (L) layer took place in 2002–2003 (10 sites; Hilli and others 2008a). Site nr. 1 in Sevettijärvi was correspondingly sampled in 2009. Missing data for understorey vegetation (one site) and litter biomass (five sites) were modelled (see Table 3). From the L layer, the following fractions could be separated: coarse tree litter; needle litter; the dead parts of dwarf shrubs, grasses, and herbs; and lower (decomposing and dead) parts of mosses and lichens (Hilli and others 2008a).
The vegetation and L layer fractions were dried at 60 °C and weighed separately. The total living biomass of the understorey vegetation per site was calculated as a sum of all species. The total mass of the L layer was obtained by summing the mass of the individual litter fractions. To determine the C concentrations, 1–8 joint samples were formed for each plant species or litter fraction in a site, depending on the plant and litter composition of the square. The site-specific carbon stock (CS) representing the whole understorey vegetation was calculated as a sum of C quantities of individual species (sum of dry weight of species fractions *C concentration). Similarly, the CS of the whole litter layer per site was calculated as a sum of C quantities of different litter fractions (dry weight of a fraction *C concentration).
Living fine and small roots of trees and understory vegetation. Fine (diameter < 2 mm) and small (diameter 2–5 mm) root samples were taken between 20 July and 12 August in 1998 as described in Merilä and others (2014). From each stand, 12 root cores were taken with a cylindrical soil corer (diameter 40 mm). The cores were divided into sections comprising the organic layer and the 0–5, 5–10, 10–20, and 20–30-cm mineral soil layers. For further information, see Helmisaari and others (2007), Merilä and others (2014), and Supplementary Material.
Soil sampling and analysis. Soil sampling included the organic layer (OFH) and mineral soil layers (depths of 0–5, 5–10, 10–20, and 20–40 cm). Organic layer samples were collected from 40 systematically located points with soil corer on the 30 m × 30 m plot and combined to provide 3–4 composite samples. Mineral soil samples were taken from 24 systematically located points by soil corer or with a shovel depending on the stoniness of the site and combined to provide 4 composite samples for each 15 study sites in 2006, as described in Merilä and others (2014) and in Lindroos and others (2022). The bulk density of the humus layer and mineral soil on the sites was determined by taking soil samples using steel cylinders (Merilä and others 2014). Soil C pools, based on the volumetric mineral soil samples, were corrected for the stone content, using the rod penetration method (Viro 1952; Tamminen 1991; Tamminen and Starr 1994). For details, see Supplementary Material.
Determination of C concentrations, calculation of C stocks. The total C concentrations of all compartments were measured with a CHN analyser (LECO). The C pool ha−1 stored in each forest ecosystem compartment (including needles, living and dead branches, stems, bark, stumps, coarse roots, living fine and small roots, understorey vegetation, litter and humus layers, and mineral soil (0–40 cm)) was calculated by multiplying the mass ha−1 of each compartment by its C concentration. For details, see Supplementary Material.
Estimation of Carbon Transfers (CT) in Tree Stand Increment, Litterfall, Cellulose Decomposition, and Water Fluxes (WF)
Stem volume increment of the stand. The diameter at breast height and tree height was measured for all trees on the site with a diameter ≥ 5.0 cm at two timepoints. The stem volumes of individual trees were computed for each tree using the KPL programme package, which computes stand and single-tree characteristics from sample plot measurements (Heinonen 1994). KPL applies the volume functions by Laasasenaho (1982). The annual stem volume increment (m−3 ha−1 y−1) was calculated as the difference between stem volume per hectare during the autumn of 2004 and spring of 2005 and the autumn of 1999 and spring of 2000, divided by the number of growing seasons between the measurements (five).
The stand volume increment was converted to increment in stem (aboveground stump stem, including bark) biomass C content using conversion factors presented by Tomppo (2000).
Litterfall (LF) (other than large branches) was collected using 12 traps located systematically on a 10 × 10 m grid on one subplot (30 × 30 m) on each Level II site stand during 1996–2007; sampling on some of the sites started later than 1996 (Ukonmaanaho and others 2008, 2016). Large LF branches that did not fit into litter bags were collected in 2007 using 12 circular traps of 0.5 m2 laid on the ground next to the litter funnels described above. Litterfall of understorey vegetation was not measured. For details of the method, see Supplementary Material.
Cellulose decomposition. As an empirical variable for decomposition rate, we determined the mass loss of bleached cellulose (softwood pulp; Lähde 1974) on the 15 sites included in this study (Table 1) and on two additional pine sites (sites number 2 and 9; see Table 2 in Merilä and Derome 2008). Cellulose strips (1 × 30 × 47 mm) were dry weighed (24 h 105 °C), singly closed into a nylon mesh bag (1 mm mesh), and transported to the field in August 2006. With the help of a rock wool knife, sixteen cellulose bags were placed at regular intervals in the interface of F and H layers on four sides of the square subplots (30 × 30 m) of each site. After an incubation of 12 months, the cellulose bags were collected in August 2007 and transported to the laboratory, where adhering material was carefully removed and washed off, after which the cellulose strips were oven-dried and weighed, as described above. The cellulose decomposition was calculated as the 1-year mass loss percentage.
Fluxes of dissolved organic carbon (DOC) in bulk deposition (BD), stand throughfall (TF), and percolation water (PW). The bulk deposition (BD), stand throughfall (TF), and soil percolation water (PW) samples were collected during 1998–2004 on the study sites (Lindroos and others 2008). However, in this study, the data were investigated in a broader context than in Lindroos and others (2008). TF samples were collected within the site by 20 deposition collectors during the snow-free period (funnel collector, diameter 200 mm) and during the winter by 6–10 snow collectors (collector ring, diam. 360 mm). The collectors were located systematically on the 30 × 30 m subplot. BD samples were collected in an open area (3 funnel collectors, 2 snow collectors) in the vicinity of the sites. PW samples were collected during the snow-free period by funnel shaped zero-tension lysimeters (diam. 200 mm) at depths of 5, 20, and 40 cm below the ground surface. Samples were collected at 2–4-week intervals (Clarke and others 2016).
The amount of precipitation in BD and TF were measured by weighing, and the water amount in PW was estimated by the anion budget method using sulphate (SO42−) as a conservative anion (Nilsson and others 1998; Lindroos and others 2008). In the calculation, the following formula was used:
where WFx = water flux (mm) at x cm depth (x = 5, 20 or 40 cm below the ground surface), SO4TF = TF sulphate concentration (mg l–1), WFTF = water flux (mm) in TF, and SO4x = sulphate concentration (mg l–1) in percolation water at a depth of x cm. The BD, TF, and PW samples were filtered through a membrane filter (0.45 µm), and the concentration of dissolved organic carbon (DOC) was determined by a TOC analyser (Shimadzu TOC5000) and SO42− by ion chromatography (IC).
Statistical Methods
We used linear mixed models to detect possible differences in C stocks and in C transfers between different compartments, tree species, and north/south locations. In the models, C stocks/transfers were dependent variables, and the compartment, species, and location north/south (and their interactions) were explanatory fixed factors. As the compartments were not (spatially) independent, compartment was defined as a repeated (within subject) factor, whereas tree species and location were between-subjects factors. As subjects (random factor), we used monitoring sites. For the repeated factor, the diagonal covariance structure was selected based on Akaike’s information criterion. The relationships between variables were investigated by means of Spearman’s rank correlation coefficient (rs).
Due to statistically significant complex interactions between the above factors (Tables S1 and S3), we dissected the analysis into compartment-wise comparisons of tree species and locations. This was done by means of general linear models with C stock/transfer as the dependent variable, and tree species and location (and their interaction) as fixed factors while monitoring site was the random factor.
Statistical analyses were performed using IBM SPSS Statistics software (ver. 22–24).
Results
Comparisons Between South and North and Between Tree Species
C Stocks (CS)
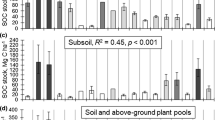
The total site C stocks (CSs) varied from 81 to 260 Mg ha−1, observed on the northernmost Scots pine site at Sevettijärvi and on the southern Norway spruce site at Evo, respectively (Table 3). Tree species, ecosystem compartment, location (north/south), and the interaction terms species*compartment and compartment*location showed a statistically significant effect on the CSs (Table S1). The major CSs were stems (30 ± 2% of total CS), mineral soil (28 ± 2%), and humus layer (13 ± 1%; Table 3, Figure. 3). Similarly to these three compartments, dead branches and stumps showed no significant difference between pine and spruce sites (Table S2). However, the interaction term Species*Location was significant for dead branches, indicating that the magnitude of difference between north and south was larger in spruce than in pine (Figure. 3). On average, the spruce sites had 40% higher CS than the pine sites. This difference was statistically significant (Table S2), but Species*Location interaction (p = 0.056) indicates it to be more apparent on southern sites (Figure. 3). Coherently, the CSs of needles, living branches, bark, and both root compartments were significantly larger in spruce than in pine sites (Tables 3 and S2, Figure. 3). However, understory plants and litter layers showed larger CS on pine than on spruce sites (Tables 3 and S2, Figure. 3).
On average, the total CSs of northern sites were 58% lower than those on the southern sites. Consistently, the CS of most compartments was lower in the north than the south. However, understorey CS was on average 2.1 times higher in the north than the south. Moreover, northern sites also had significantly more fine and small root C than southern sites (Tables 3 and S2, Figure. 3). Only humus layer CS showed no significant difference between northern and southern sites (Table S2).
The difference in total CS between northern and southern sites was more distinct among spruce sites, the CS being on average c. two times higher in the south than the north. This was primarily due to a larger stem volume and thus CS in southern stands, though the southern spruce sites showed higher CS for most compartments than on the northern sites (Table 3, Figure. 3).
The C ratio between above- and belowground tree compartments was significantly lower in the north than the south (Table S2). At the ecosystem level, the aboveground C/belowground C ratio showed no significant differences between north and south (p = 0.140) or between tree species.
C in stand Increment, Transfers, and Water Fluxes
Quantity of carbon in the annual increment of stem volume was significantly lower in the north than the south (Tables 4 and S4). Similarly, the annual LF CT (carbon transfer via litterfall), sampled with funnels (needles and other LF), traps on the ground (large branches), or both, showed no significant differences between tree species, and was significantly smaller in the north than the south (Table S4). In the case of total funnel LF, the interaction term Species*Location was significant, indicating a larger difference between north and south on spruce than pine sites (Tables 4 and S4). For total LF, this term was tentatively significant.
The flux of dissolved organic carbon (DOC) in bulk deposition (BD) was significantly lower in the north than the south (Table S4). In throughfall (TF), the DOC flux was higher on spruce than pine sites and higher in the south than the north; the interaction term Species*Location was also significant, indicating that the difference in TF DOC flux between the south and north was greater in spruce than in pine (Tables 4 and S4). DOC fluxes in soil percolation water at depths of 5, 20, and 40 cm showed no significant differences between tree species or between north and south (Table S4).
Cellulose mass loss percentage was significantly lower in the north than the south; this difference was somewhat greater on pine sites than on spruce sites, but not quite significantly (Species*Location interaction: p = 0.101; Figure. 4, Tables S4, S5).
The Relationships Between C Stocks, C Transfers, and Water DOC Fluxes
All aboveground tree compartment CSs, mineral soil CS, and total CS were positively correlated (rS = 0.51–0.89, p ≤ 0.054, n = 15; Table S6) with litterfall CT components (needle LF, total funnel LF, large branch LF, and the sum of all LF components). The correlations of LF CTs with understory vegetation CS were negative (rS = − 0.88 to − 0.80, p < 0.01) and remained nonsignificant with CSs of live fine and small roots, litter layer, and humus layer.
On average, annual LF CTs, that is, needle LF, total LF captured by funnels, large branches, and the sum of all LF components, were 1.6 ± 0.13%, 2.7 ± 0.18%, 0.4 ± 0.05%, and 3.1 ± 0.22% (mean ± S.E.) of the stem C stock, respectively. These proportions showed no significant differences between tree species or between north and south; the only exception was large branch LF (north < south, p = 0.027). The proportions of the respective LF CTs from the total aboveground tree CS were all significantly higher in the south than the north (0.007 ≤ p < 0.056).
Generally, DOC in throughfall (TF) was positively correlated with the aboveground tree compartment CSs (rS = 0.57–82, p < 0.03). As DOC in bulk deposition (BD) decreases from south to north similarly to tree CSs, DOC in BD was also positively correlated with some of the tree compartment CSs, for example, with stem CS (rS = 0.58, p = 0.023).
DOC fluxes in percolation water (depths 5, 20, and 40 cm) were not significantly correlated with any of the measured CSs. However, DOC fluxes in the north (depth 20 cm) were significantly correlated with live branch, bark, and live fine and small root CS (rS = 0.94, p = 0.005, n = 6). In addition, DOC flux at a depth of 40 cm was correlated with needle LF (rS = 0.812, p = 0.050, n = 6).
The cellulose mass loss percentage was negatively correlated with latitude (rS = − 0.56, p = 0.03, n = 15) and with live fine and small root CS (rS = − 0.60, p = 0.017).
C/N Ratio of the Organic Layer: Relationships with Latitude, C Transfers, and C Stocks
The C/N ratio of the organic layer (C/Norg), often used as an index of site fertility, was significantly higher in the north (p < 0.001) than the south and higher on the pine sites than the spruce sites (p = 0.052). C/Norg was also positively correlated with latitude (rS = 0.61, p = 0.016). C/Norg was negatively correlated with most CSs and CTs; the highest correlation appeared with the total CS (rS = − 0.90, p < 0.001) and throughfall DOC flux (rS = –0.91, p < 0.001). C/Norg was positively correlated with understorey vegetation (rS = 0.86, p < 0.001).
Discussion
Carbon Stocks (CS) and Allocation to Different Ecosystem Compartments
In our study, forest ecosystem C stocks decreased northwards, which may be attributed to climate, the main driver of biomass production. The C/N ratio of the organic layer (C/Norg), often used as an index of site fertility (for example, Tamminen 2000), was higher in the north and on the pine sites, having lower site fertility than spruce sites. Thus, both the variation in climatic conditions and the differences in site fertility were reflected in the organic layer C/N ratio which negatively correlated with most CSs and CTs, indicating the N limitation of the ecosystems. However, the relationship between CSs and the C/N ratio is poorly represented in soil carbon and ecosystem models, which may result in spatial discrepancies and underestimation of modelled soil carbon stocks (Hashimoto and others 2017; Ťupek and others 2016).
Basically, our results on C stocks and transfers were of the same order of magnitude with those in previous studies, for example, by Berggren Kleja and others (2008) and Hansson and others (2013). The largest CSs in our study sites were stems (30 ± 2%), mineral soil (0–40 cm; 28 ± 2%), and humus layer (13 ± 1%). Our study sites were located in mature stands and had on average 65% and 116% higher stem volume than the mean stand volumes in northern and southern Finland, respectively (Merilä and others 2014; Korhonen and others 2021). However, stem volumes of our forest sites (145 ± 90 and 310 ± 146 m3 ha–1 for northern and southern sites, respectively) corresponded well to those given by National Forest Inventory for mature managed forest sites in northern (147 m3 ha–1) and southern (284 m3 ha–1) Finland (Natural Resources Institute Finland 2023). It should be noted that our southern sites included Norway spruce-dominated protected forest in Evo (nr. 19) having exceptionally high stem volume (650 m3 ha−1).
Our results for C stocks, with the previous results on N stocks (Merilä and others 2014), reveal the importance of mineral soil (0–40 cm layer) as a major long-term storage of organic C and N (c. 70% of total N; Merilä and others 2014) in boreal coniferous forest ecosystems. The accumulation of C in mineral soil results from a positive balance between organic matter input (organic matter production and consequent above- and belowground litterfall) and output (decomposition and leaching) within the forest ecosystem. As discussed in Merilä and others (2014), podzolisation processes drive the stratification of C in mineral soil, resulting in the accumulation of dissolved organic matter in the enrichment layer (B-horizon) of the podzolic soil profile (for example, Lindroos and others 2008). Besides root litter, soil-forming processes have a strong influence on the composition of organic carbon in mineral soil layers and consist of resistant, slowly decomposing humic substances (Rumpel and others 2002; Hilli and others 2012).
According to Kauppi and others (1997), the average C stock in humus layer and mineral soil of upland forests in Finland was 72 000 kg ha−1 in the top 75 cm layer. In our study, the mean C stock in the organic layer and mineral soil (0–40 cm) ranged 39 000–123 000 kg ha−2. Moreover, in our study sites the average proportion of C stock in the organic layer from the total soil stock was 31% (mineral soil layer 0–40 cm), while the respective value reported by Liski and Westman (1997) for Finland was 28% (mineral soil layer 0–100 cm). This comparison indicates that soil C stocks of our study sites were somewhat above national average, which is most probably due to higher fertility of our sites compared to national average (see also Lindroos and others 2022). The greatest change in the C stock is considered to take place in the organic layer and topmost mineral soil (Gaudinski and others 2000). However, as deeper mineral soil possesses a large C stock, its role in the C cycling would deserve more attention and examination.
After stems and mineral soil, humus layer formed the third largest C stock (13% of total), being higher on spruce than pine sites. Also, the total C stocks were largest on spruce sites, especially in the south. As site fertility was higher on spruce sites than pine sites, the pure effect of tree species on the C sequestration in the humus layer and the whole ecosystem remained untested. However, in a study including data from four decades of mean humus layer thickness in > 82 000 plots, Berg and others (2009) reported that in similar site conditions, pine sequestered more C than spruce (difference of 71 kg C ha−1y−1), indicating the importance of pine forests for C sequestration. Especially, Scots pine is adapted to conditions of low moisture and nutrient availability showing higher volume production than Norway spruce in such conditions (Ilvessalo 1927; Heiskanen and Mäkitalo 2002; Ekö and others 2008).
Interestingly, humus layer was the only compartment showing no significant difference in C stocks between north and south despite harsher climate and lower fertility in the north. Although the mean humus layer C stock within the spruce sites was higher in the south than the north, the difference remained nonsignificant because of the large variation within the southern sites. In addition to the balance between sequestration and losses of C, stabilisation of soil organic matter (SOM) leading to protection of organic matter from mineralization plays a major role in the formation of humus layer and its C storage. Higher C/N ratio in the humus layer of our northern sites compared to southern ones suggests higher recalcitrance of SOM (Nohrstedt 1985), which thus might be one factor explaining nonsignificant differences in humus layer CSs between north and south, even though both chemically resistant and more labile compounds are known to form stable SOM (Cotrufo and others 2015). Furthermore, Hilli and others (2008a, b) who used a subset of our study sites found that the northern sites showed lower concentrations of the recalcitrant fraction (acid insolubles) of organic matter and higher concentrations of more easily degradable fractions (water-soluble extractives and nonpolar extractives) than the southern sites, presumably due to the lower decomposition rate in the northern climate. Altogether, SOM stabilisation is a result of many factors (Clemmensen and others 2013; Cotrufo and others 2015; Liang and others 2017) and cannot be explained only by recalcitrance of plant necromass or climate (Adamczyk 2021).
The allocation of C, as well as N (Merilä and others 2014), to roots was proportionally higher in the north than the south. This is in line with the results of Leppälammi-Kujansuu and others (2014), who found that Norway spruce stands in the northern boreal region produced relatively more litter below- than aboveground. These results reflect the lower fertility and lower density of northern forests, where the resource competition for nutrients is more severe than for light. In contrast, the canopy interception of light is much higher in southern spruce forests and thus controls the resource availability of the understorey more severely (Valladares and others 2016), which is reflected in the lowest understorey C stocks on these sites. There, the understorey vegetation community also includes more herbs (and grasses) with lower biomass than perennial dwarf shrubs, which are more abundant on less fertile pine sites and northern spruce sites.
The opposite response of understory vegetation to site fertility in comparison with tree C stocks reflects the control of the tree canopy on the resource availability of understory vegetation. Lehtonen and others (2016) estimated that in eastern and northern Finnish Lapland the understorey litterfall equals c. half of the total litter input and concluded that ecosystem modelling and greenhouse gas inventory schemes should elaborate estimates of understorey litter production in the northern latitudes. Further, Leppälammi-Kujansuu and others (2014) found that on northern boreal Norway spruce sites, understorey litter may form up to a third of the total annual aboveground litter production and nearly a quarter of that of belowground litter production. Results showing that sites with lower fertility produced relatively more belowground litter were also reported for Scots pine (Ding and others 2021) on a site fertility gradient in southern Finland. In those Scots pine stands, the understorey production contributed 18–41% of belowground production.
The data for the ecosystem components were collected in different years during 1996–2009. For example, root sampling occurred in 1998, tree stand measurements during dormant period in 2004–2005, and sampling for understorey vegetation in 2009. Annual variation in the biomass of different ecosystem compartment studied evidently exists but its effect on our results is difficult to quantify. In case of litterfall and percolation water CTs however, we used long-term means. Moreover, as our study sites represent mature tree stands the variation may not be very substantial. For example, fine-root biomass in tree stands has been found to reach a steady state in mature stands (Vogt and others 1987; Claus and George 2005).
Five of the sites were slightly thinned after the dormant season 2004–2005 after measurements for calculation of stand biomass were carried out (see Material and Methods). The thinnings may have had a minor effect on the results of litterfall (calculated as mean 1996–2007) and understorey vegetation (sampled in 2009), as their sampling period occurred at least partly after the thinnings. However, no drastic changes were noticeable in these variables in comparison with pre-thinning years.
Tree-related biomass estimates were based on modelling and thus bring some inaccuracy to our results. However, the input data of diameter at breast height, tree height, and crown length applied in biomass estimation originate from detailed tree measurements carried out on the study sites. Moreover, the biomass functions applied are developed for Finnish conditions and are based on extensive empirical data (Repola 2009).
C Transfers (CT) and Their Relationship with C Stocks
The quantity of C in the annual increment of stump stem volume (increment C, m3 ha−1 y−1) and the annual litterfall CT (needles and other LF, and large branches) were significantly lower in the north than the south. This is in line with biomass amounts in different ecosystem parts, indicating more favourable conditions in southern Finland, with a warmer climate than in northern Finland (for example, Liski and Westman 1997; Merilä and others 2014).
A corresponding northing effect was also reflected in the cellulose mass loss percentage, the flux of dissolved organic carbon (DOC) in bulk deposition (BD), and throughfall (TF), which were lower in the north than the south. In addition, TF DOC flux was higher on spruce than pine sites. Many complex processes prevail in the soil, and this probably masks the northing and tree species effects on soil percolation water, as the DOC fluxes in soil percolation water showed no differences between tree species or between north and south.
Most carbon stock compartments were significantly correlated with litterfall CT components. Litterfall is major aboveground C transfer from the forest vegetation into the soil. Indeed, the key factors to be considered in any soil C models are litter production and decomposition processes (for example, Ťupek and others 2019).
Generally, the DOC flux in TF was positively correlated with the aboveground tree compartment CSs, while DOC fluxes in percolation water were not correlated with any of the measured CSs. However, in the north, the DOC fluxes in the percolation water of the topsoil at rooting depth (depth 20 cm) were positively correlated, for example, with fine and small root CS. The harsh climate and shortage of plant-available N in northern forests is shown to be related to the expansion and maintenance of the ectomycorrhizal root biomass and length (Ostonen and others 2011). Thus, DOC inputs originating from their decomposition and exudates may significantly contribute to the DOC fluxes in percolation water.
Conclusions
Our comprehensive study of the distribution of carbon stocks and transfers within boreal forest ecosystems provided an improved insight into the absolute and relative magnitude of the C stocks and transfers, as well as their mutual relationships. The study revealed patterns in the stocks and transfers of forest carbon, which were mainly driven by site fertility and climatic variation along the latitudinal gradient between 60 and 69 degrees. Our field observations demonstrated that site fertility was crucial for boreal forest CSs, calling for a re-evaluation of soil C and Earth system future projections in a warming climate and forest management. Furthermore, our results may serve basic and applied research, as well as practical forest management, in considering the carbon sequestration capacity of boreal forests.
Data availability
Dataset including variables used in this study is available in the Supplementary Material.
Change history
28 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10021-024-00921-0
References
Adamczyk B. 2021. How do boreal forest soils store carbon? BioEssays 43:2100010.
Ahti T, Hämet-Ahti L, Jalas J. 1968. Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5:169–211.
Andivia E, Rolo V, Jonard M, Formánek P, Ponetter Q. 2016. Tree species identity mediates mechanisms of top soil carbon sequestration in a Norway spruce and European beech mixed forest. Ann For Sci 73:437–47.
Berg B, Johansson MB, Nilsson A, Gundersen P, Norell L. 2009. Sequestration of carbon in the humus layer of Swedish forests – direct measurements. Can J For Res 39:962–75.
Berggren Kleja D, Svensson M, Majdi H, Jansson P-E, Langvall O, Bergkvist B, Johansson MB, Weslien P, Truusb L, Lindroth A, Ågren GI. 2008. Pools and fluxes of carbon in three Norway spruce ecosystems along a climatic gradient in Sweden. Biogeochemistry 89:7–27.
Clarke N, Zlindra D, Ulrich E, Mosello R, Derome J, Derome K, König N, Lövblad G, Draaijers GPJ, Hansen K, Thimonier A, Waldner P. 2016. Part XIV: Sampling and Analysis of Deposition. UNECE ICP Forests Programme Co-ordinating Centre, editor. Manual on methods and criteria for harmonized sampling assessment monitoring and analysis of the effects of air pollution on forests. Thünen Institute of Forest Ecosystems Eberswalde Germany. pp 66
Claus A, George E. 2005. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can J For Res 35:1617–25.
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD. 2013. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 340:1615–18.
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ. 2015. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–9.
Dawud SM, Raulund-Rasmussen K, Domisch T, Finer L, Jaroszewicz B, Vesterdal L. 2016. Is tree species diversity or species identity the more important driver of soil carbon stocks C/N Ratio and pH? Ecosystems 19:645–60.
Ding Y, Leppälammi-Kujansuu J, Minkkinen K, Kulmala L, Salemaa M, Schiestl-Aalto P, Ukonmaanaho L, Nöjd P, Merilä P, Helmisaari H-S. 2021. Distinct patterns of below- and aboveground growth phenology and litter carbon inputs along a boreal site type gradient. For Ecol Manag 489:119081.
Ekö PM, Johansson U, Petersson N, Bergqvist J, Elfving B, Frisk J. 2008. Current growth differences of Norway spruce (Picea abies), Scots pine (Pinus sylvestris) and birch (Betula pendula and Betula pubescens) in different regions in Sweden. Scand J For Res 23:307–18.
Fernández-Martinez M, Vicca S, Janssens IA, Sardans J, Luyssaert S, Campioli M, Chapin FS III, Ciais P, Malhi Y, Obersteiner M, Papale D, Piao SL, Reichstein M, Rodá F, Peñuelas J. 2014. Nutrient availability as the key regulator of global forest carbon balance. Nat Clim Change 4:471–6.
Fierer N, Craine JM, Mclauchlan K, Schimel JP. 2005. Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326.
Gaudinski JB, Trumbore SE, Davidson EA, Zheng S. 2000. Soil carbon cycling in a temperate forest: radio-carbon-based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry 51:33–69.
Hansson K, Fröberg M, Helmisaari H-S, Berggren Kleja D, Olsson BA, Olsson M, Persson T. 2013. Carbon and nitrogen pools and fluxes above and below ground in spruce, pine and birch stands in southern Sweden. For Ecol Manag 309:28–35.
Hashimoto S, Nanko K, Ťupek B, Lehtonen A. 2017. Data-mining analysis of the global distribution of soil carbon in observational databases and Earth system models. Geosci Model Dev 10:1321–37.
Heinonen J. 1994. Koealojen puu- ja puustotunnusten laskentaohjelma KPL. Käyttöohje. Summary. Computer program package for computing stand and single tree characteristics from sample plot measurements. Metsäntutkimuslaitoksen Tiedonantoja 504:1–80.
Heiskanen J, Mäkitalo K. 2002. Soil water-retention characteristics of Scots pine and Norway spruce forest sites in Finnish Lapland. For Ecolo Manag 162:137–52.
Helmisaari H-S, Derome J, Nöjd P, Kukkola M. 2007. Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol 27:1493–1504.
Hilli S, Stark S, Derome J. 2008a. Carbon quality and stocks in organic horizons in boreal forest soils. Ecosystems 11:270–82.
Hilli S, Stark S, Derome J. 2008b. Qualitative and quantitative changes in water-extractable organic compounds in the organic horizon of boreal coniferous forests. Boreal Environ Res 13(Suppl. B):107–19.
Hilli S, Stark S, Willför S, Smeds A, Reunanen M, Hautajärvi R. 2012. What is the composition of AIR? Pyrolysis-GC-MS characterization of acid-insoluble residue from fresh litter and organic horizons under boreal forests in southern Finland. Geoderma 179–180:63–72.
Ilvessalo Y. 1927. Suomen metsät. Tulokset vuosina 1921–1924 suotitetusta valtakunnan metsien arvioimisesta. [The forests of Finland. Results of the general survey of the forests of the country carried out during the years 1921–1924]. Metsätieteellisen Koealaitoksen Julkaisuja 11: 1–421 [+ 192 pp]. [In Finnish with English summary].
Kauppi PE, Posch M, Hänninen P, Henttonen HM, Ihalainen A, Lappalainen E, Starr M, Tamminen P. 1997. Carbon reservoirs in peatlands and forest in the boreal regions of Finland. Silva Fenn 31(1):13–25.
Korhonen KT, Ahola A, Heikkinen J, Henttonen HM, Hotanen JP, Ihalainen A, Melin M, Pitkänen J, Räty M, Sirviö M, Strandström M. 2021. Forests of Finland 2014–2018 and their development 1921–2018. Silva Fenn 55:10662.
Laasasenaho J. 1982. Taper curve and volume functions for pine spruce and birch. Commun Inst For Fenn 108:74.
Laganière J, Cavard X, Brassard BW, Paré D, Bergeron Y, Chen HYH. 2015. The influence of boreal tree species mixtures on ecosystem carbon storage and fluxes. For Ecol Manag 354:119–29.
Lähde E. 1974. Rate of decomposition of cellulose in forest soils in various parts of the Nordic countries. Rep Kevo Subarct Res Stn 11:72–8.
Lehtonen A, Linkosalo T, Peltoniemi M, Sievänen R, Mäkipää R, Tamminen P, Salemaa M, Nieminen T, Ťupek B, Heikkinen J, Komarov A. 2016. Soil carbon stock estimates in a nationwide inventory: evaluating performance of the ROMUL and Yasso07 models. Geosci Model Dev 9:4169–83.
Leppälammi-Kujansuu J, Salemaa M, Kleja DB, Linder S, Helmisaari H-S. 2014. Fine root turnover and litter production of Norway spruce in a long-term temperature and nutrient manipulation experiment. Plant Soil 374:73–88.
Liang C, Schimel JP, Jastrow JD. 2017. The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105.
Lindroos A-J, Derome J, Mustajärvi K, Nöjd P, Beuker E, Helmisaari H-S. 2008. Fluxes of dissolved organic carbon in stand throughfall and percolation water in 12 boreal coniferous stands on mineral soils in Finland. Boreal Environ Res 13(Suppl. B):22–34.
Lindroos A-J, Mäkipää R, Merilä P. 2022. Soil carbon stock changes over 21 years in intensively monitored boreal forest stands in Finland. Ecol Indic 144:109551.
Liski J, Wetman CJ. 1997. Carbon storage in forest soil of Finland. 2. Size and regional patterns. Biogeochemistry 36(3):261–74.
Lorenz M, Fischer R. 2013. Pan-European forest monitoring: an overview. In: Ferretti M, Fischer R, Eds. Forest Monitoring – Methods for terrestrial investigations in Europe with an overview of North America and Asia. Developments in Environmental Science. Vol. 12. Amsterdam: Elsevier Ltd. pp 19–32.
Melvin AM, Mack MC, Johnstone JF, McGuire AD, Genet H, Schuur EAG. 2015. Differences in ecosystem carbon distribution and nutrient cycling linked to forest tree species composition in a mid-successional boreal forest. Ecosystems 18:1472–88.
Merilä P, Derome J. 2008. Relationships between needle nutrient composition in Scots pine and Norway spruce stands and the respective concentrations in the organic layer and in percolation water. Boreal Environ Res 13(suppl. B):35–47.
Merilä P, Mustajärvi K, Helmisaari HS, Hilli S, Lindroos A-J, Nieminen TM, Nöjd P, Rautio P, Salemaa M, Ukonmaanaho L. 2014. Above- and below-ground N stocks in coniferous boreal forests in Finland: Implications for sustainability of more intensive biomass utilization. For Ecol Manag 311:17–28. https://doi.org/10.1016/j.foreco.2013.06.029.
Nanko K, Hashimoto S, Miura S, Ishizuka S, Sakai Y, Levia DF, Ugawa S, Nishizono T, Kitahara F, Osone Y, Kaneko S. 2017. Assessment of soil group site and climatic effects on soil organic carbon stocks of topsoil in Japanese forests. Eur J Soil Sci 68:547–58.
Natural Resources Institute Finland. 2023. Statistics database/forest statistics/forest resources/mean growing stock volume on forest land available for wood production by stand development classes (m3/ha). Available at https://statdb.luke.fi/PxWeb/pxweb/en/LUKE/LUKE__04%20Metsa__06%20Metsavarat/1.23_Puuston_keskitilavuus_puuntuotannon_met.px/ [Cited 2023-08-08]
Nilsson SI, Berggren D, Westling O. 1998. Retention of deposited NH4+-N and NO3–N in coniferous forest ecosystems in southern Sweden. Scand J For Res 13:393–401.
Nohrstedt H-Ö. 1985. Biological activity in soil from stands in Central Sweden, as related to site properties. Microbial Ecol 11:259–266.
Ortiz CA, Liski J, Gärdenes AI, Lehtonen A, Lundblad M, Stendahl J, Ågren GI, Karltun E. 2013. Soil organic carbon stock changes in Swedish forest soils – A comparison of uncertainties and their sources through a national inventory and two simulation models. Ecol Model 251:221–31.
Ostonen I, Helmisaari H-S, Borken W, Tedersoo L, Kukumägi M, Bahram M, Lindroos A-J, Nöjd P, Uri V, Merilä P, Asi E, Löhmus K. 2011. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob Change Biol 17:3620–32.
Pan YD, Birdsey RA, Fang JY, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao SL, Rautiainen A, Sitch S, Hayes D. 2011. A large and persistent carbon sink in the world’s forests. Science 333:988–93.
Rautio P, Fürst A, Stefan K, Raitio H, Bartels U. 2020. Part XII: Sampling and Analysis of Needles and Leaves. Version 2020–3. UNECE ICP Forests Programme Co-ordinating Centre, editor. Manual on methods and criteria for harmonized sampling assessment monitoring and analysis of the effects of air pollution on forests. Thünen Institute of Forest Ecosystems Eberswalde Germany. pp 16. + Annex [http://www.icp-forests.org/Manual.htm].
Repola J. 2009. Biomass equations for Scots Pine and Norway Spruce in Finland. Silva Fenn 43:625–47.
Repola J, Ojansuu R, Kukkola M. 2007. Biomass functions for Scots pine Norway spruce and birch in Finland. Working papers of the finnish forest research institute. Vol. 53. pp 28
Rumpel C, Kogel-Knabner I, Bruhn F. 2002. Vertical distribution age and chemical composition of organic carbon in two forest soils of different pedogenesis. Org Geochem 33:1131–42.
Salemaa M, Derome J, Nöjd P. 2008. Response of boreal forest vegetation to the fertility status of the organic layer along a climatic gradient. Boreal Environ Res 13(Suppl. B):48–66.
Salemaa M, Hamberg L. 2007. Understorey vegetation on the Level II plots during 1998–2004. Working papers of the finnish forest research institute. Vol. 45. pp 69–80
Shanin V, Komarov A, Mäkipää R. 2014. Tree species composition affects productivity and carbon dynamics of different site types in boreal forests. Eur J For Res 133:273–86.
Tamminen P. 2000. Soil factors. In: Mälkönen E, Ed. Forest Condition in a Changing Environment – The Finnish Case. Forestry Sciences. Vol. 65. Dordrecht: Kluwer Academic Publishers. pp 72–86.
Tamminen P, Starr M. 1994. Bulk density of forested mineral soils. Silva Fenn 28:53–60.
Tamminen P. 1991. Kangasmaan ravinnetunnusten ilmaiseminen ja viljavuuden alueellinen vaihtelu Etelä-Suomessa. [Expression of soil nutrient status and regional variation in soil fertility of forested site in southern Finland]. Folia Forestalia. Vol. 777 . pp 40. [In Finnish with English summary].
Todd-Brown KEO, Post Randerson JT., WM, Hoffman FM, Tarnocai C, Schuur EAG, Allison SD. 2013. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 10:1717–36.
Tomppo E. 2000. National forest inventory of Finland and its role estimating the carbon balance of forests. Biotechnol Agron Soc Environ 4:281–4.
Ťupek B, Ortiz CA, Hashimoto S, Stendahl J, Dahlgren J, Karltun E, Lehtonen A. 2016. Underestimation of boreal soil carbon stocks by mathematical soil carbon models linked to soil nutrient status. Biogeosciences 13:4439–59.
Ťupek B, Launiainen S, Peltoniemi M, Sievänen R, Perttunen J, Kulmala L, Penttilä T, Lindroos A-J, Hashimoto S, Lehtonen A. 2019. Evaluating CENTURY and Yasso soil carbon models for CO2 emissions and organic carbon stocks of boreal forest soil with Bayesian multi-model inference. Eur J Soil Sci 70:847–58.
Ukonmaanaho L, Merilä P, Nöjd P, Nieminen TM. 2008. Litterfall production and nutrient return to the forest floor in Scots pine and Norway spruce stands in Finland. Boreal Environ Res 13(Suppl. B):67–91.
Ukonmaanaho L, Pitman R, Bastrup-Birk A, Breda N, Rautio P. 2016. Part XIII: Sampling and Analysis of Litterfall. UNECE ICP Forests Programme Co-ordinating Centre, editor. Manual on methods and criteria for harmonized sampling assessment monitoring and analysis of the effects of air pollution on forests. Thünen Institute for Forests Ecosystems Eberswalde Germany. pp 15. http://www.icpforests.org/Manual.htm.
Valladares F, Laanisto L, Niinemets U, Zavala MA. 2016. Shedding light on shade: ecological perspectives of understorey plant life. Plant Ecol Divers 9:237–51.
Vesterdal L, Clarke N, Sigurdsson BD, Gundersen P. 2013. Do tree species influence soil carbon stocks in temperate and boreal forests? For Ecol Manag 309:4–18.
Viro PJ. 1952. Kivisyyden määrittämisestä. [On the determination of stoniness]. Communicationes Institute Forestalis Fenniae, Vol. 40. pp 23. [In Finnish with English summary].
Vogt KA, Vogt DJ, Moore EE, Fatuga BA, Redling MR, Edmonds RL. 1987. Conifer and angiosperm fine-root biomass in relation to stand age and site productivity in Douglas fir forests. J Ecol 75:857–70.
Acknowledgements
Besides Natural Resources Institute Finland’s resources, part of the data utilised in the study was collected with co-funding provided within the frameworks of the EU/Forest Focus (EC) No. 2152/2003 and EU/Life+ FutMon programmes. Boris Ťupek and Päivi Merilä received co-funding from the LIFE+ financial instrument of the European Union (LIFE12 ENV/FI/000409 Monimet) and PM and Antti-Jussi Lindroos also from the Finnish Ministry of Agriculture and Forestry (MaaTi project, PI Raisa Mäkipää). We thank Kaisa Mustajärvi for help in the calculation of the results and Aleksi Lehtonen and Mikko Peltoniemi for comments and discussions on the manuscript. The contribution of field and laboratory staff is gratefully acknowledged.
Funding
Open access funding provided by Natural Resources Institute Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author's contributions: PM, A-JL, TMN, PR, MS, and LU conceived the study; PM, A-JL, H-SH, SH, TMN, PN, PR, MS, BT, and LU performed research, and PM, A-JL, H-SH, SH, PN, PR, MS, and LU analysed data. PM compiled the results. PM led writing the paper, for which all co-authors contributed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merilä, P., Lindroos, AJ., Helmisaari, HS. et al. Carbon Stocks and Transfers in Coniferous Boreal Forests Along a Latitudinal Gradient. Ecosystems 27, 151–167 (2024). https://doi.org/10.1007/s10021-023-00879-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00879-5