Abstract
Many vegetated coastal ecosystems are formed through ecosystem engineering by clonal vegetation. Recent work highlights that the spatial shoot organization of the vegetation determines local sediment accretion and subsequently emerging landscape morphology. While this key engineering trait has been found to differ between species and prevailing environmental conditions, it remains unknown how the interplay of both factors drive shoot organization and therefore landscape morphology. Here, we compared the spatial shoot organization of young, clonally expanding plants of the two dominant European dune grass species: sand couch (Elytrigia juncea) and marram grass (Ammophila arenaria) across a range of coastal dune environments (from Denmark to France). Our results reveal that, on average, sand couch deployed a more dispersed shoot organization than marram grass, which has a patchy (Lévy-like) organization. Whereas sand couch exhibited the same expansion strategy independent of environmental conditions, marram grass demonstrated a large intraspecific variation which correlated to soil organic matter, temperature and grain size. Shoot patterns ranged from a clumped organization correlating to relatively high soil organic matter contents, temperature and small grain sizes, to a patchy configuration with intermediate conditions, and a dispersed organization with low soil organic matter, temperature and large grain size. We conclude that marram grass is flexible in adjusting its engineering capacity in response to environmental conditions, while sand couch instead follows a fixed expansion strategy, illustrating that shoot organization results from the interaction of both species-specific and environmental-specific trait expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Co-occurring dune grasses have different shoot organizations
-
Shoot organization is more flexible in marram grass than in sand couch
-
Dune grasses’ engineering traits are both species- and environment-dependent
Introduction
Vegetated coastal ecosystems such as coastal dunes, salt marshes and seagrass beds are among the most productive ecosystems in the world and provide important goods and services, including flood protection, carbon sequestration, biodiversity enhancement and tourism (Burke and others 2001; Martínez and others 2007; Barbier and others 2011). The emergence and maintenance of these ecosystems depend on the interaction between sediment stabilization by vegetation and sediment transport by flows of wind or water (Jones and others 1994; Corenblit and others 2011; Balke 2013). With its physical structures, the vegetation attenuates wind or water flow, causing airborne or water-suspended sediments to settle. In turn, sedimentation (and other plant induced changes) can feed back to the plant’s trait expression, such as shoot elongation or vertical rhizome development, making these systems feedback driven (Maun 1998; Hacker and others 2019). The extent to which flows are reduced depends on plant structural traits, such as shoot density, flexibility and length (Hacker and others 2012; Bouma and others 2013). Generally, higher sedimentation rates are associated with dense, inflexible and tall vegetation (Bouma and others 2013; Goldstein and others 2017; Hacker and others 2019; Mullins and others 2019). However, short vegetation can have a higher local sediment trapping compared to a more downwind sediment accumulation of tall vegetation (Hesp and others 2019). Consequently, variation in structural traits of individual plants can affect large-scale landscape morphology (Baas and Nield 2007; Corenblit and others 2015; Schwarz and others 2018; Hacker and others 2019).
Coastal dunes occur along wave-dominated sandy shores and protect about one-third of the world’s shoreline (Martínez and others 2007; Durán and Moore 2013). Dune grasses are the main ecosystem engineering species responsible for building coastal dune landscapes (Feagin and others 2015). It has long been recognized that shoot density is an important structural trait determining the engineering capacity of dune grasses (for example, Hesp 1989; Zarnetske and others 2012; Hacker and others 2019). In dense vegetation, local sedimentation rates are high, but area colonization is slow resulting in high and narrow dunes. In contrast, sparse vegetation leads to rapid colonization but low local sedimentation rates, resulting in lower and broader dunes (Hesp 1989; Hacker and others 2012, 2019; Zarnetske and others 2012). Shoot density, and consequently dune shape, is commonly presented as a species-specific trait (Zarnetske and others 2012; Goldstein and others 2017; Hacker and others 2019). Thus far, most field studies have concentrated on comparing differences in shoot densities and their effects in established dune grasses that already formed (embryonic) dunes. However, differences in structural traits in the initial stages of beach colonization can be at least as important because they potentially have a stronger effect on dune formation by controlling plant survival and sand capture from the start.
Recently, it was found that young, establishing dune grass individuals of two species of Ammophila (American beachgrass (Ammophila breviligulata) and marram grass (Ammophila arenaria)), rather than having a uniform or random shoot organization, deploy a more patchy clonal expansion strategy (Reijers and others 2019b). Strikingly, their shoot organization could be well described by heavy-tailed random walk models in which many smaller steps are alternated by an occasional longer step, that are commonly used to describe optimization in animal search behaviour (Reijers and others 2019b). The two beach grass species were found to employ somewhat different clonal expansion strategies. Supporting experiments revealed that these clonal expansion strategies allowed the plants to balance plant expansion and sediment accretion (Reijers and others 2019b). American beachgrass displayed the most dispersed strategy, which was associated with the highest overall sediment accretion over a large area (that is, maximized total entrapped sand volume). The more patchy organization of marram grass instead maximized dune building efficiency (that is, investment in clonal growth versus entrapped sand volume) (Reijers and others 2019b). Follow-up work in two contrasting environments and under experimental conditions highlighted that marram grass shifts its shoot placement strategy depending on sediment availability. When deprived of sediment the plant exhibited a clumped, single-patch organization, whereas the characteristic patchy organization emerged in response to sediment burial (Reijers and others 2021). While our previous work demonstrates adaptability of shoot organizations in contrasting environments, a continuous gradient in environmental conditions was lacking. Moreover, whether co-occurring dune building grasses deploy similar expansion strategies in the same environment or adapt comparably to changes in sediment availability remains unknown.
In this study, we investigated 1) how the clonal expansion strategy differs between two co-occurring dune grass species with contrasting dune shapes: sand couch (Elytrigia juncea) and marram grass (Ammophila arenaria), and 2) how local environmental conditions (that is, soil organic matter, nutrients levels, grain size, distance to sea, temperature, precipitation and wave conditions) affect trait expression of both species by comparing individuals of the same species along the Northwestern European coast (Denmark–France). Sand couch and marram grass are native, often co-occurring European species and are the dominant dune building species along the Northwestern European coast (Figure S1). Both species have a rhizomatous clonal growth, with marram grass having the ability to create vertical and horizontal rhizomes while sand couch only creates horizontal rhizomes (Huiskes 1979; Harris and Davy 1986a). Generally, marram grass relies on establishment from rhizomal fragments on the beach/foredune interface (Huiskes 1977), whereas for sand couch establishment from seeds and from clonal fragments both occur (Harris and Davy 1986a). Usually, sand couch grows closer to the sea than marram grass, where it initiates dune building by creating low and broad dunes, after which marram grass colonizes and forms higher and more narrow dunes (van Puijenbroek and others 2017c; Reijers and others 2019a). Generally, the high, narrow dunes of marram grass are associated with high resistance, while the lower, broader dunes of sand couch potentially build a more resilient landscape, similar to pioneer species on the US east coast (Feagin and others 2015; Zinnert and others 2017). The species have some distinct differences in their morphology and physiological tolerance, with sand couch having a higher salt tolerance, shorter shoot length and lower shoot density (Bakker 1976; van Puijenbroek and others 2017c; Reijers and others 2019a).
We hypothesize that in general, sand couch displays a more dispersed shoot pattern than marram grass, corresponding to the observed differences in dune morphologies (Figure S1). We expect both species to have context-dependent shoot organizations, but eco-evolutionary mechanisms behind the shoot organizations to be different. Specifically, we expect marram grass to change from a clumped to patchy organization with increase in sediment supply (Reijers and others 2021), which is generally higher on wide, dissipative beaches (Delgado-Fernandez 2010; Walker and others 2017). In contrast, we hypothesize that sand couch’s shoot organization is mostly affected by nutrient levels, especially by nitrogen which is generally the most limiting resource in the beach/dune landscape (Willis 1965; Kachi and Hirose 1983; Reijers and others 2019a). This hypothesis follows earlier findings on Elymus mollis, the pioneer dune grass of the US west coast, that invests more in long rhizomes (dispersed shoot organization) under higher nitrogen levels than marram grass (Pavlik 1983). Here, we expect a similar response in sand couch with a more dispersed organization at locations with higher nitrogen levels.
Methods
Field Survey
We determined the clonal expansion strategies of sand couch (Elytrigia juncea) and marram grass (Ammophila arenaria) at nine locations along the Northwestern European coast (Fig. 1). The sampling sites were visited once during the growing season of 2019 (June–October) and were selected for their presence of young, establishing vegetation on the beach/foredune interface where sand is directly supplied from the foreshore. Except for the island of Griend, all selected locations were publicly accessible. However, no beaches with recreational facilities (for example, restaurants or sports) were selected. Furthermore, all locations are Natura 2000 areas except the dunes near Lemvig (Denmark). These systems have a wide variety in physical conditions such as beach width (ranging from 30 to 800 m, beach width strongly correlated (r = 0.99) with plant-to-sea distance which is used hereafter) and significant wave height (ranging from 0.3 to 1.1 m) (Table S1). Out of these nine sites, young, establishing patches of sand couch and marram grass co-occurred at three sites. At three sites, only isolated patches of establishing sand couch were present, and in the remaining three only isolated patches of marram grass were found. At all locations, both species were present in later successional stages.
To determine the plant’s clonal expansion strategy, we followed methods as described by Reijers and others (2019b). In brief, we selected isolated establishing plants at each location (Table S1). From each clonal individual, we cut off and replaced all shoots with a labelled pin. The coordinates of shoots (in cm) were extracted from calibrated still images (between 100*100 cm and 150*150 cm) using a custom-made MATLAB tool (Reijers and Hoeks 2019). Subsequently, the step size distribution of each individual plant was determined by connecting all individual shoots of the clonal individual using a nearest neighbour connection algorithm. Each individual was excavated to verify rhizomal connections between the shoots (that is, confirm that the selected vegetation patch consists of one clonal individual). For characterization of the clonal expansion strategy, we included only those individuals where connections between all shoots were confirmed.
In addition, we determined four other plant traits—shoot length, shoot diameter, tissue nitrogen content and C/N ratio—from each plant. Prior to cutting of the shoots, the length and diameter of five randomly selected shoots were measured. We collected leaf tissue (pooled per clonal individual) to assess leaf nitrogen and carbon levels. After freeze-drying the leaves, they were ground using a ball mill (MM400, Retch Haan, Germany). Using about 1 mg of the homogenized sample, C and N concentrations were determined using an elemental analyser (Carlo Erba NA1500, Thermo Fisher Scientific, Waltham, MA, USA).
Finally, we obtained eight environmental variable that characterize each site: organic matter, grain size distribution, sediment total N, orthophosphate, significant wave height, distance to sea (that is, beach width), average annual temperature and precipitation. Grain size, organic matter, and plant available nitrogen and phosphorus were determined in soil samples taken from between the roots. Grain size was determined in a freeze-dried subsample of the soil using a laser diffraction and polarization intensity differential scattering technology with a particle size analyser (Coulter LS 13 320). Soil organic matter was expressed as loss on ignition (4 h, 550 °C). Salt extracts were taken using 17.5 g fresh soil in 50 ml of 0.2 M NaCl. In the extracts, nitrogen and phosphorus levels were determined using an AutoAnalyzer 3 system (Brand and Luebbe, Norederstedt, Germany or Skalar and Seal autoanalyzer). Distance to sea was used as a proxy of beach width, and general sand supply (Delgado-Fernandez 2010; Walker and others 2017), and was retrieved for each individual plant. Significant wave height was obtained at the level of each location (sources see Table S2), and mean annual temperature and precipitation were retrieved from the closest official weather station for the period 2016–2019 (from local meteorological institutes, sources see Table S2).
Characterizing Clonal Expansion Strategies
To characterize the clonal expansion strategy, the step size distribution was determined for each individual using a nearest neighbour connecting algorithm which was previously validated for marram grass by Reijers and others (2029b). This algorithm consecutively searches the nearest neighbour until all shoots (N) are connected and selects the shortest possible route among N iterations to derive aboveground distances (step sizes) between shoots. The expansion strategy was determined for the individuals with sufficient step size data (over 30 connections). Smaller plants were discarded from further analyses. Five commonly used random walk models for describing movements were used to describe the observed step size distributions: an exponential (Brownian), a two-mode exponential (Composite Brownian), a log-normal, a power-law (Lévy) and a truncated power-law (truncated Lévy) (see Supplement for a detailed description of different models). We used a Kolmogorov–Smirnov (KS) test to assess whether the observed step sizes were significantly different from the fitted distributions (more info in Supplement). Models were compared with each other using weighted AIC values (Wagenmakers and Farrell 2004).
Individuals were compared using the scaling exponent (µ) of the truncated Lévy model, as this model was never rejected by the KS test, and thus fitted acceptably to all individuals (Table S3). Thus, for every included individual plant, the observed step size distribution was not significantly different from a truncated Lévy distribution. The probability density function of the truncated Lévy distribution is given by
For each individual, the minimum step size (smin) was estimated using KS statistics (Clauset and others 2009). For some of the plants, the estimated smin led to a large loss of steps (> 33% loss or < 30 steps remaining); in these cases, the measured minimal step size was used as smin with a fixed minimum of 0.68 cm, which is twice the measuring error calculated from translating pixels to cm (~ 0.34 cm). The scaling component (μ) was determined using maximum-likelihood estimator given that
and
with n being the number of shoots and s being the step size. The scaling component of the truncated Lévy is an indication of the shoot organization with lower values indicating a higher proportion of large step sizes (that is, a more dispersed growth).
Statistical Analysis
Model fitting, validation and verification were done in MATLAB (2020, The Mathworks, Inc.). Further statistical analyses were performed in R (version 3.6.1). First, a general impression of clonal expansion strategies of both species was made using pooled step size data of all individuals. Second, plant traits (that is, µ exponent of the truncated Lévy, shoot length and diameter, leaf nitrogen and C/N ratio) were compared between locations and species using ANOVA combined with Fisher’s least significant difference (LSD) post hoc tests. In addition, the variance in plant traits between both species was tested with a Levene’s test. For every test, normality of the residuals was checked and, if needed, the data were transformed using log transformation. P values lower than 0.05 were considered statistically significant.
Principle component analysis of environmental variables (that is, soil organic matter, median grain size, total soil nitrogen and phosphate, distance to sea, average annual temperature and precipitation and significant wave height) was used to determine which environmental variables were most important in differentiating locations. All variables were averaged per location, centred and standardized to account for different units of the measured variables. Subsequently, it was tested whether the first two principal components correlated with plant traits for both species. Furthermore, the correlation between the individual environmental variables and plant traits was tested. As there were multiple non-normally distributed factors, we based the correlations on Spearman’s rank correlation.
Results
Interspecific variation in traits
Clonal expansion strategies. Sand couch had a more dispersed shoot organization (that is, relatively more large steps) than marram grass as showed by the pooled step size data (Fig. 2, Figure S2). On average, the expansion strategies of both sand couch and marram grass were best described by heavy-tailed distributions, indicating a patchy strategy that most strongly resembled a Composite Brownian (sand couch) and a truncated Lévy (marram grass) (Fig. 2). The heavy-tailed distributions (Composite Brownian, Lévy and truncated Lévy) were also the model expansion strategies that best fitted most individual plants (89% of the sand couch individuals and 91% of marram grass individuals) (Fig. 2, Table S3). However, the variation in best fitting models was larger for marram grass than for sand couch, with most marram grass individuals resembling a Lévy distribution (57%), followed by a truncated Lévy (26%) and Composite Brownian (9%). For sand couch, a truncated Lévy distribution was the best fit for 75% of the plants. This indicates a larger variation in shoot organizations within marram grass compared to sand couch. Overall, the truncated Lévy distribution was the model that fitted most individuals (48% in both species combined) and was not rejected for any of the individuals based on KS statistics (Table S3). On average, the µ exponent of the truncated Lévy for sand couch individuals was 1.52 ± 0.038, which reflects a dispersed distribution (Fig. 3, Figure S3). Marram grass had a significantly higher µ exponent reflecting a patchier shoot organization (2.05 ± 0.070, F1,61 = 38.49, P < 0.001, Fig. 3, Figure S3).
a The clonal growth strategy showed as the inverse cumulative frequency distribution of the pooled step sizes of marram grass (Ammophila arenaria, blue point data, 2875 steps) and sand couch (Elytrigia juncea, red point data, 2211 steps). The slope of the sand couch data is less steep than the one of marram grass, representing a larger number of longer steps in sand couch and thus a more dispersed growth. b Percentage of best fitting random walk models per species based on weighted AIC values. The numbers within the bars represent the number of individuals with the corresponding best fitting random walk distribution. Most of the plants (of both species) displayed a heavy-tailed distribution (Composite Brownian, Truncated Lévy or Lévy).
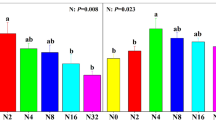
The clonal expansion strategies of sand couch (E. juncea) and marram grass (A. arenaria) found along the European coast with; a A conceptual depiction of how shoot organization relates to the µ exponent of the truncated Lévy distribution and b the found µ exponents of sand couch and marram grass at sampled locations, showing a larger variation in clonal growth strategies of marram grass than sand couch. The horizontal bars depict median value, box height the first and third quartile and whiskers the minimum and maximum values. Locations are ordered from north to south (left to right). Letters depict LSD post hoc grouping (P < 0.05).
Other traits. On average, sand couch had shorter shoots than marram grass (28.0 ± 1.7 vs 48.1 ± 2.1 cm, respectively, F(1,44) = 33.30, P < 0.001, Figure S4), with a similar shoot diameter (2.7 ± 0.08 (marram grass) vs 2.5 ± 0.1 (sand couch) mm, F(1,44) = 3.61, Figure S4). Nitrogen levels in the leaf tissue of sand couch were significantly higher than in marram grass (30.5 ± 1.4 mg g−1 (sand couch) vs 17.3 ± 0.7 mg g−1 (marram grass), F(1,48) = 83.11, P < 0.001, Figure S4), which also led to a lower C/N ratio in sand couch (16.5 ± 0.9 (sand couch) vs 28.3 ± 1.3 (marram grass), F(1,48) = 73.15, P < 0.001, Figure S4).
Intraspecific Variation in Traits
The variation in shoot organization was much larger for marram grass than for sand couch (Levene’s test, F1,61 = 10.9, P = 0.002, Fig. 3, Figure S5). Sand couch displayed a similar shoot organization across locations, while marram grass ranged from a clumped configuration on Griend (µ = 2.51 ± 0.10) to a dispersed, sand couch-like, organization on Rømø (µ = 1.67 ± 0.11, Fig. 3, Figure S3). Furthermore, the variance in leaf nitrogen levels was larger in sand couch than in marram grass (ranging from 1.9 ± 0.1% at Skagen to 3.9 ± 0.1% at Lemvig for sand couch and 1.5 ± 0.2% at Sylt to 2.0 ± 0.2% at Texel in marram grass, Levene’s test, F1, 58 = 9.90, P = 0.003, Figure S4). The variance of shoot length and diameter was similar for both species but did differ between locations (Figure S4).
Correlation of Intraspecific Traits With Environmental Variables
In the principal component analysis, axis 1 explained 38.8% of the environmental variation between locations with soil organic matter (r = 0.94), significant wave height (r = − 0.84) and average annual temperature (r = 0.74) being the most highly weighted variables (Fig. 4, Table S4). Axis 2 explained 18.1% with grain size (r = 0.71), temperature (r = 0.62) and precipitation (r = − 0.49) being most influential (Fig. 4, Table S4). No significant correlations between PC1, PC2 or individual environmental variables and shoot organization of sand couch were found. For marram grass, PC1 correlated significantly with the scaling exponent and shoot length (Table 1). From the individual environmental variables, soil organic matter and average annual temperature correlated positively to the scaling component of marram grass (r = 0.60, P < 0.001 and r = 0.36, P = 0.04, respectively, Table 1), indicating a shift from dispersed to a clumped organization with increasing soil organic matter and temperature. Median grain size correlated negatively (r = − 0.43, P = 0.01), indicating a more dispersed organization with increase in grain size (Table 1).
Principal component analysis including averaged environmental variables per location. Soil organic matter (OM), significant wave height (wave) and temperature were the most important for PC1, which explained 38.8% of the variation. Grain size (grain) and precipitation were most influential for PC2, which explained 18.1% of the variation.
For both species, plant traits correlated to multiple environmental variables (Table 1). For marram grass, shoot length had the strongest correlation with soil organic matter and grain size (similar to shoot organization; r = 0.58, P < 0.001 and r = − 0.43 P = 0.01, respectively). Furthermore, the shoot diameter was most strongly correlated with distance to sea and significant wave height (r = 0.47, P = 0.006 and r = − 0.46, P = 0.02, respectively) while no correlations between foliar nitrogen or C/N ratio and environmental variables were found. For sand couch, shoot length correlated significantly with soil phosphate level and grain size (r = − 0.57, P = 0.003 and r = 0.67, P < 0.001, respectively), shoot diameter correlated to soil organic matter (r = 0.46, P = 0.02), and foliar N level and C/N ratio correlated to soil nitrogen content (r = 0.43, P = 0.03 and r = − 0.48, P = 0.01, respectively).
Discussion
In our comparison of clonal expansion strategies of dune grasses across Northwestern Europe, we found that heavy-tailed expansion strategies dominate for both species. Thus, most individuals deploy a strategy that deviates from a simple dense vegetation patch and display a more patchy shoot organization, balancing expansion rate and sediment capturing efficiency (Reijers and others 2019b). However, pattern characteristics differed between both species with sand couch demonstrating a more dispersed shoot organization associated with sand capture over a large area, while the patchier organization of marram grass is associated with high local sand-capturing efficiency (Reijers and others 2019b). Whereas marram grass expressed intraspecific variation in clonal expansion strategy, from dispersed to clumped which correlated with soil organic matter, temperature and grain size, contrary to our expectations, sand couch demonstrated very little variation. These findings demonstrate that shoot organization is not solely a species-specific or environmental-specific trait, but instead depends on the interaction of these two variables. Hence, our study highlights the need to study key traits for ecosystem engineering species and associated engineering strength across a range of environmental conditions and through time to understand the reciprocal interactions between trait expression, environmental conditions and the ecosystem engineering capacity.
Relation Between Interspecific Variation in Structural Traits and Dune Morphologies
The two dominant dune building grasses of Western Europe are associated with different dune morphologies. Whereas dunes formed by sand couch remain relatively low (max ± 3 m), they are much broader than the high (max ± 20 m) dunes formed by marram grass (Bakker 1976; van Puijenbroek and others 2017b, Figure S1). Similar to previous studies comparing North American beach grasses (Hacker and others 2019), we found clear differences between the species’ structural traits (for example, shoot length and shoot pattern) that are associated with differences in sand-capturing ability (Zarnetske and others 2012; Reijers and others 2019b). The dispersed growth of sand couch promotes sand capture over a large area, consequently building relatively low and broad dunes, while the patchier growth strategy of marram grass is associated with higher local sand trapping efficiency, promoting a taller and narrow dune form (Reijers and others 2019b). Additionally, sand couch had on average a lower shoot length which is associated with less per-shoot flow reduction and thus less sediment accretion (Hesp 1989; Van Dijk and others 1999). This suggests that in similar conditions marram grass has locally higher sedimentation rates, leading to the emergence of higher, but steeper dune profiles than sand couch (Figure S1).
As a result of the difference in shoot organization and length, it is likely that changes of flooding are higher for sand couch than for marram grass in similar conditions during dune development, from plant establishment to foredune formation. However, as sand couch is more salt tolerant, it is less vulnerable to flooding (Sykes and Wilson 1989; van Puijenbroek and others 2017c). Instead, flooding may even be beneficial for sand couch as nutrients are transported to the beach during overwash (Reijers and others 2019a). In contrast, marram grass is known to perform well in nutrient-poor conditions as the species can recycle its own material through litter-decomposition feedbacks and thus requires less allochthonous material transported during overwash events to grow (Kooijman and Besse 2002). In line with this, we found that foliar nitrogen levels of sand couch correlated with soil nitrogen levels, while no significant correlation was found in marram grass. Overwash events do not only transport material from the sea to the coast (for example, sediment and nutrients) as wave run-ups during storm surges can cause massive dune erosion (Vellinga 1982; Haerens and others 2012; van Puijenbroek and others 2017a). Whereas marram grass can vertically outgrow high burial rates by building a strong underground network of roots and rhizomes that bind sand and resist erosion, the more sparsely growing sand couch outgrows accumulated sand through shoot elongation leaving the dune body more vulnerable to erosion (Feagin and others 2015; Konlechner and others 2016; van Puijenbroek and others 2017a). However, the higher nutrient use efficiency causes sand couch to exhibit a faster recovery and higher recolonization potential than marram grass which makes this species less vulnerable to erosion events on a population level (Harris and Davy 1986b; Sykes and Wilson 1990; van der Putten 1990; Reijers and others 2019a).
Based on the observed differences in species’ structural traits (for example, shoot length and organization) and dune morphologies, we hypothesize that sand couch has evolved towards building a dune landscape that balances the risks of flooding and erosion (that is, dislodgement, osmotic stress) with its potential benefits (that is, nutrients, low burial), whereas we expect that marram grass has evolved to maximize sand capture to escape flooding completely. In that light, the so-called pioneer species sand couch could have adopted a dispersed clonal expansion strategy associated with rapid expansion and high resilience, whereas the patchy strategy of marram grass promotes high local engineering associated with building resistance, but lower resilience (Reijers and others 2019a). To disentangle such feedback relationships, we suggest that plant trait expression, physical conditions, vegetation-sedimentation feedbacks and coastal morphodynamics need to be monitored in the field over the course of multiple years.
Intraspecific Variation in Clonal Expansion Strategies of Different Dune Grass Species
We expected both species to express variation in clonal expansion strategy in response to their environment, with marram grass responding to sediment supply (that is, with increase in distance to sea) and sand couch to nitrogen levels. However, whereas the clonal expansion strategy of marram grass clearly differed between locations, the strategy of sand couch hardly varied (Fig. 3, Figure S5). Contrary to our expectations, we found no correlation between distance to sea (that is, beach width) and the shoot organization of marram grass and no response of sand couch to differences in soil nitrogen levels. The latter finding seemingly contrasts with earlier experimental studies on an American pioneer dune grass that found that lower nitrogen levels promote biomass allocation to the rhizomes to escape nutrient stress and increase landscape colonization rates (Pavlik 1983). However, our values are similar or even lower than the low nitrogen levels used in the experimental study, which were described as “nutrient stress”. Although we found more variation in leaf nitrogen levels for sand couch than marram grass (from 1.7% to 4.2% vs 1.0% to 2.7%), we report no variation in shoot organization for sand couch.
In line with previous findings (Reijers and others 2021) for marram grass, we found a clear variation in the clonal expanding strategy, ranging from clumped (max µ exponent of 2.9) to dispersed (min µ exponent of 1.2) with on average a Lévy-like expansion strategy. Although our previous work showed that a trait shift in expansion strategy could be related to different sediment supply rates—with a clumped strategy being the most efficient at low sedimentation rates and a patchy to dispersed strategy at higher sedimentation rates (Reijers and others 2021)—in our current study we find no correlation with distance to sea, which was used as a proxy for sediment availability (Delgado-Fernandez 2010; Walker and others 2017). Instead, we found that the scaling component was positively correlated with soil organic matter and temperature and negatively correlated with grain size (Table 1).
Generally, higher soil organic matter levels and sediment with a smaller grain size are found in more sheltered locations that experience less disturbance and are associated with lower sedimentation rates (Incera and others 2003; Nylén and others 2015). On these locations—with a relatively high soil organic matter content and small grain size—marram grass displayed a more clumped shoot organization (that is, a higher scaling exponent). Another explanation for the positive correlation between soil organic matter and shoot clumping may be that retention of water, which is a scarce resource in these sandy systems, increases with increase in soil organic matter content. As a result, the local environment becomes increasingly favourable causing the plant to place new shoots in close proximity (Rawls and others 2003). Furthermore, higher temperatures were correlated with a more clumped organization. However, previous studies found no correlation between dry mass production or tillering and temperature in marram grass (Huiskes 1979; Biel and Hacker 2021). It is possible that the found positive correlation is rather an effect of site selection than of temperature (that is, more sheltered locations were selected at lower latitudes), additional field observations are needed to disentangle these effects.
Other included environmental factors did not correlate with observed differences in clonal expansion strategy. However, some correlations with shoot length and diameter were found. No clear pattern in environmental variables and these plant traits between species was visible (or even contrasting correlations were found). As the age of the individuals and some environmental variables (for example, elevation or overwash history) were unknown, climatic events such as storms or droughts that might impact plant traits could not be included. Additionally, for some of the included variables the spatiotemporal resolution (Table S2) might be too coarse to assess the relation between individual trait expression and local environmental conditions. Next to variation in environmental conditions, the unexplained variation in clonal expansion may be caused by genetic variation between individuals and locations (Rodríguez-Echeverría and others 2008). Little is known about the genetic variation within and between marram grass populations and the relation between trait expression and genetic variation. Future research that includes genetic diversity could disentangle the effects of environmental variability and genetic variation on trait expression in landscape forming species. Overall, our results demonstrate that the expression of this key ecosystem engineering trait is dependent on species identity and is relatively fixed for sand couch, but flexible for marram grass. Therefore, we emphasize that trait-based approaches and biogeomorphic models that use mean trait values derived from trait databases should be aware of potential intraspecific differences in trait expression (Brückner and others 2019; De Battisti and others 2019). Furthermore, we argue that extensive field sampling with repeated measures to link vegetation growth patterns and landscape morphodynamics is essential to understand the complex interactions between multiple factors including species identify, genetic background, environmental conditions and individual age that together steer trait expression.
Implications for Restoration
Marram grass has been introduced around the world to stabilize drifting sand and fortify coastal landscapes (for example, Hertling and Lubke 1999; Gadgil 2002; Rozé and Lemauviel 2004; Nordstrom 2021). Within its native range, our survey demonstrated that environmental conditions impact its spatial organization, while planting designs are, irrespective of environmental conditions with 30 to 60 cm spacing between individuals, in dispersed competition-limiting arrays (van der Putten 1990). Over the last decades, emphasis on including intraspecific facilitation (that is, changing to a clumped, facilitation maximizing design) in restoration of vegetated coastal ecosystems increased (Silliman and others 2015; Sofawi and others 2017; Fischman and others 2019; Temmink and others 2020). While many of these studies stress the importance of intraspecific facilitation, our results imply that the optimal planting design is context-dependent (van der Heide and others 2021) and species-specific (Reijers and others 2019b, 2021). Our results suggest that for marram grass, restoration efficacy could be increased by understanding more in depth the relation between plant organization and physical conditions. For sand couch, our work predicts that the optimal planting design would be dispersed–but still heavy-tailed patchy–irrespective of the location.
Outside its native range, marram grass plantings have drastically altered coastal dune environments by changing dune morphology, reducing sediment transport to the hinterland and lowering local biodiversity (for example, in New Zealand, South Africa and USA) (Hertling and Lubke 1999; Gadgil 2002; Pickart 2021). Dunes planted with marram grass generally grow higher, more stable and homogeneous. Although these high dune landscapes formed by marram grass are resistant—by withstanding and mitigating storm surges—the lower dunes that are formed by faster growing native grasses might be more resilient as they possibly have higher recovery potential after erosive events (Pickart 2021). By planting ecosystem engineering species with different structural traits or trait-environment relationships, coastal landscapes might lose their natural resilience (Feagin and others 2015; Schwarz and others 2016; Hsu and Stallins 2020; Pickart 2021). Therefore, inclusion of other species in restoration projects, such as sand couch for Northwestern Europe, may result in faster dune formation and a more heterogeneous landscape with potentially a higher resilience.
Data Availability
Data are freely available and can be obtained at https://doi.org/10.25850/nioz/7b.b.0d.
References
Baas ACW, Nield JM. 2007. Modelling vegetated dune landscapes. Geophysical Research Letters 34.
Bakker JP. 1976. Phytogeographical Aspects of the Vegetation of the Outer Dunes in the Atlantic Province of Europe. Journal of Biogeography 3:85.
Balke T. 2013. Establishment of biogeomorphic ecosystems: A study on mangrove and salt marsh pioneer vegetation.[S.l.: s.n.].
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81:169–193.
De Battisti D, Fowler MS, Jenkins SR, Skov MW, Rossi M, Bouma TJ, Neyland PJ, Griffin JN. 2019. Intraspecific Root Trait Variability Along Environmental Gradients Affects Salt Marsh Resistance to Lateral Erosion. Frontiers in Ecology and Evolution 7.
Biel RG, Hacker SD. 2021. Warming alters the interaction of two invasive beachgrasses with implications for range shifts and coastal dune functions. Oecologia 197:757–770.
Bouma TJ, Temmerman S, van Duren LA, Martini E, Vandenbruwaene W, Callaghan DP, Balke T, Biermans G, Klaassen PC, van Steeg P, Dekker F, van de Koppel J, de Vries MB, Herman PMJ. 2013. Organism traits determine the strength of scale-dependent bio-geomorphic feedbacks: A flume study on three intertidal plant species. Geomorphology 180:57–65.
Brückner MZM, Schwarz C, van Dijk WM, van Oorschot M, Douma H, Kleinhans MG. 2019. Salt Marsh Establishment and Eco-Engineering Effects in Dynamic Estuaries Determined by Species Growth and Mortality. Journal of Geophysical Research: Earth Surface 124:2962–2986.
Burke L, Kura Y, Kassem K, Revenga C, Spalding M, McAllister D, Caddy J. 2001. Coastal ecosystems. Washington: World Resources Institute Washington, DC.
Clauset A, Shalizi CR, Newman MEJ. 2009. Power-law distributions in empirical data. SIAM Review 51:661–703.
Corenblit D, Baas ACW, Bornette G, Darrozes J, Delmotte S, Francis RA, Gurnell AM, Julien F, Naiman RJ, Steiger J. 2011. Feedbacks between geomorphology and biota controlling Earth surface processes and landforms: A review of foundation concepts and current understandings. Earth-Science Reviews 106:307–331.
Corenblit D, Baas A, Balke T, Bouma T, Fromard F, Garófano-Gómez V, González E, Gurnell AM, Hortobágyi B, Julien F, Kim D, Lambs L, Stallins JA, Steiger J, Tabacchi E, Walcker R. 2015. Engineer pioneer plants respond to and affect geomorphic constraints similarly along water-terrestrial interfaces world-wide. Global Ecology and Biogeography 24:1363–1376.
Delgado-Fernandez I. 2010. A review of the application of the fetch effect to modelling sand supply to coastal foredunes. Aeolian Research 2:61–70.
Durán O, Moore LJ. 2013. Vegetation controls on the maximum size of coastal dunes. Proceedings of the National Academy of Sciences of the United States of America 110:17217–17222.
Feagin RA, Figlus J, Zinnert JC, Sigren J, Martínez ML, Silva R, Smith WK, Cox D, Young DR, Carter G. 2015. Going with the flow or against the grain? The promise of vegetation for protecting beaches, dunes, and barrier islands from erosion. Frontiers in Ecology and the Environment 13:203–210.
Fischman HS, Crotty SM, Angelini C. 2019. Optimizing coastal restoration with the stress gradient hypothesis. Proceedings of the Royal Society B 286:20191978.
Gadgil RL. 2002. Marram grass (Ammophila arenaria) and coastal sand stability in New Zealand. New Zealand Journal of Forestry Science 32:165–180.
Goldstein EB, Moore LJ, Vinent OD. 2017. Lateral vegetation growth rates exert control on coastal foredune ‘hummockiness’ and coalescing time. Earth Surface Dynamics 5:417–427.
Hacker SD, Zarnetske P, Seabloom E, Ruggiero P, Mull J, Gerrity S, Jones C. 2012. Subtle differences in two non-native congeneric beach grasses significantly affect their colonization, spread, and impact. Oikos 121:138–148.
Hacker SD, Jay KR, Cohn N, Goldstein EB, Hovenga PA, Itzkin M, Moore LJ, Mostow RS, Mullins EV, Ruggiero P. 2019. Species-specific functional morphology of four US atlantic coast dune grasses: Biogeographic implications for dune shape and coastal protection. Diversity 11:82.
Haerens P, Bolle A, Trouw K, Houthuys R. 2012. Definition of storm thresholds for significant morphological change of the sandy beaches along the Belgian coastline. Geomorphology 143:104–117.
Harris D, Davy AJ. 1986b. Strandline Colonization by Elymus Farctus in Relation to Sand Mobility and Rabbit Grazing. The Journal of Ecology:1045–56.
Harris D, Davy AJ. 1986a. Regenerative Potential of Elymus Farctus From Rhizome Fragments and Seed. The Journal of Ecology 74:1057.
Hertling UM, Lubke RA. 1999. Use of Ammophila arenaria for dune stabilization in South Africa and its current distribution - Perceptions and problems. Environmental Management 24:467–482.
Hesp PA. 1989. A review of biological and geomorphological processes involved in the initiation and development of incipient foredunes. Proceedings of the Royal Society of Edinburgh, Section b: Biological Sciences 96:181–201.
Hesp PA, Dong Y, Cheng H, Booth JL. 2019. Wind flow and sedimentation in artificial vegetation: Field and wind tunnel experiments. Geomorphology 337:165–182.
Hsu L-C, Stallins JA. 2020. Multiple representations of topographic pattern and geographic context determine barrier dune resistance, resilience, and the overlap of coastal biogeomorphic models. Annals of the American Association of Geographers 110:640–660.
Huiskes AHL. 1977. The Natural Establishment of Ammophila arenaria from Seed. Oikos.
Huiskes AHL. 1979. Ammophila Arenaria (L.) Link (Psamma Arenaria (L.) Roem. et Schult.; Calamgrostis Arenaria (L.) Roth). The Journal of Ecology.
Incera M, Cividanes SP, Lastra M, López J. 2003. Temporal and spatial variability of sedimentary organic matter in sandy beaches on the northwest coast of the Iberian Peninsula. Estuarine, Coastal and Shelf Science 58:55–61.
Jones CG, Lawton JH, Shachak M. 1994. Organisms as Ecosystem Engineers. Ecosystem management, . Springer, New York: New York. pp 130–147.
Kachi N, Hirose T. 1983. Limiting nutrients for plant growth in coastal sand dune soils. The Journal of Ecology:937–44.
Konlechner TM, Orlovich DA, Hilton MJ. 2016. Restrictions in the sprouting ability of an invasive coastal plant, Ammophila arenaria, from fragmented rhizomes. Plant Ecology 217:521–532.
Kooijman AM, Besse M. 2002. The higher availability of N and P in lime-poor than in lime-rich coastal dunes in the Netherlands. Journal of Ecology 90:394–403.
Martínez ML, Intralawan A, Vázquez G, Pérez-Maqueo O, Sutton P, Landgrave R. 2007. The coasts of our world: Ecological, economic and social importance. Ecological Economics 63:254–272.
MATLAB. 2020. Version 9.9.0 (R2020b). The MathWorks Inc., Natick, Massachusetts.
Maun MA. 1998. Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany 76:713–738.
Mullins E, Moore LJ, Goldstein EB, Jass T, Bruno J, Durán Vinent O. 2019. Investigating dune-building feedback at the plant level: Insights from a multispecies field experiment. Earth Surface Processes and Landforms 44:1734–1747.
Nordstrom KF. 2021. Beach and dune restoration. Cambridge University Press.
Nylén T, Hellemaa P, Luoto M. 2015. Determinants of sediment properties and organic matter in beach and dune environments based on boosted regression trees. Earth Surface Processes and Landforms 40:1137–1145.
Pavlik BM. 1983. Nutrient and Productivity Relations of the Dune Grasses Ammophila arenaria and Elymus mollis. III. Spatial Aspects of Clonal Expansion with Reference to Rhizome Growth and the Dispersal of Buds. Bulletin of the Torrey Botanical Club 110:271–279.
Pickart AJ. 2021. Ammophila Invasion Ecology and Dune Restoration on the West Coast of North America. Diversity 13:629.
Rawls WJ, Pachepsky YA, Ritchie JC, Sobecki TM, Bloodworth H. 2003. Effect of soil organic carbon on soil water retention. Geoderma 116:61–76.
Reijers VC, Lammers C, de Rond AJA, Hoetjes SCS, Lamers LPM, van der Heide T. 2019a. Resilience of beach grasses along a biogeomorphic successive gradient: resource availability vs. clonal integration. Oecologia 192:201212.
Reijers VC, Siteur K, Hoeks S, van Belzen J, Borst ACW, Heusinkveld JHT, Govers LL, Bouma TJ, Lamers LPM, van de Koppel J, van der Heide T. 2019b. A Lévy expansion strategy optimizes early dune building by beach grasses. Nature Communications 10:1–9.
Reijers VC, Hoeks S, van Belzen J, Siteur K, de Rond AJA, van de Ven CN, Lammers C, van de Koppel J, van der Heide T. 2021. Sediment availability provokes a shift from Brownian to Lévy-like clonal expansion in a dune building grass. Ecology Letters 24:258–268.
Reijers VC, Hoeks S. 2019. Assessing the clonal expansion strategy of landscape-forming plants.
Rodríguez-Echeverría S, Freitas H, Van Der Putten WH. 2008. Genetic diversity and differentiation of Ammophila arenaria (L.) link as revealed by ISSR markers. Journal of Coastal Research 24:122–126.
Rozé F, Lemauviel S. 2004. Sand Dune Restoration in North Brittany, France: A 10-Year Monitoring Study. Restoration Ecology 12:29–35.
Schwarz C, Ysebaert T, Vandenbruwaene W, Temmerman S, Zhang L, Herman PMJ. 2016. On the potential of plant species invasion influencing bio-geomorphologic landscape formation in salt marshes. Earth Surface Processes and Landforms 41:2047–2057.
Schwarz C, Gourgue O, van Belzen J, Zhu Z, Bouma TJ, van de Koppel J, Ruessink G, Claude N, Temmerman S. 2018. Self-organization of a biogeomorphic landscape controlled by plant life-history traits. Nature Geoscience 11:672–677.
Silliman BR, Schrack E, He Q, Cope R, Santoni A, Van Der Heide T, Jacobi R, Jacobi M, Van De Koppel J. 2015. Facilitation shifts paradigms and can amplify coastal Restoration efforts. Proceedings of the National Academy of Sciences 112:14295–14300.
Sofawi AB, Rozainah MZ, Normaniza O, Roslan H. 2017. Mangrove rehabilitation on Carey Island, Malaysia: an evaluation of replanting techniques and sediment properties. Marine Biology Research 13:390–401.
Sykes MT, Wilson JB. 1989. The effect of salinity on the growth of some New Zealand sand dune species. Acta Botanica Neerlandica 38:173–182.
Sykes MT, Wilson JB. 1990. An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand. Acta Botanica Neerlandica 39:171–181.
Temmink RJM, Christianen MJA, Fivash GS, Angelini C, Boström C, Didderen K, Engel SM, Esteban N, Gaeckle JL, Gagnon K. 2020. Mimicry of emergent traits amplifies coastal restoration success. Nature Communications 11:1–9.
van der Putten WH. 1990. Establishment of Ammophila arenaria (marram grass) from culms, seeds and rhizomes. Journal of Applied Ecology 27:188–199.
van der Heide T, Temmink RJM, Fivash GS, Bouma TJ, Bostrom C, Didderen K, Unsworth RKF, Christianen MJA. 2021. Coastal restoration success via emergent trait-mimicry is context dependent. Biological Conservation 264:109373.
Van Dijk PM, Arens SM, Van Boxel JH. 1999. Aeolian processes across transverse dunes. II: Modelling the sediment transport and profile development. Earth Surface Processes and Landforms: the Journal of the British Geomorphological Research Group 24:319–333.
van Puijenbroek MEB, Limpens J, de Groot AV, Riksen MJPM, Gleichman M, Slim PA, van Dobben HF, Berendse F. 2017a. Embryo dune development drivers: beach morphology, growing season precipitation, and storms. Earth Surface Processes and Landforms 42:1733–1744.
van Puijenbroek MEB, Nolet C, De Groot AV, Suomalainen JM, Riksen MJPM, Berendse F, Limpens J. 2017b. Exploring the contributions of vegetation and dune size to early dune development using unmanned aerial vehicle (UAV) imaging. Biogeosciences 14:5533–5549.
van Puijenbroek MEB, Teichmann C, Meijdam N, Oliveras I, Berendse F, Limpens J. 2017c. Does salt stress constrain spatial distribution of dune building grasses Ammophila arenaria and Elytrichia juncea on the beach? Ecology and Evolution 7:7290–7303.
Vellinga P. 1982. Beach and dune erosion during storm surges. Coastal Engineering 6:361–387.
Wagenmakers EJ, Farrell S. 2004. AIC model selection using Akaike weights. Psychonomic Bulletin and Review 11:192–196.
Walker IJ, Davidson-Arnott RGD, Bauer BO, Hesp PA, Delgado-Fernandez I, Ollerhead J, Smyth TAG. 2017. Scale-dependent perspectives on the geomorphology and evolution of beach-dune systems. Earth-Science Reviews 171:220–253.
Willis AJ. 1965. The influence of mineral nutrients on the growth of Ammophila arenaria. The Journal of Ecology:735–45.
Zarnetske PL, Hacker SD, Seabloom EW, Ruggiero P, Killian JR, Maddux TB, Cox D. 2012. Biophysical feedback mediates effects of invasive grasses on coastal dune shape. Ecological Society of America 93:1439–1450.
Zinnert JC, Stallins JA, Brantley ST, Young DR. 2017. Crossing scales: The complexity of barrier-island processes for predicting future change. BioScience 67:39–52.
Acknowledgements
We thank Renske Veldhuis for her help in the field and laboratory and Marcel van der Meer, Ronald van Bommel, Evaline van Weerlee and Jan van Ooijen for their help with laboratory analyses. This study was financially supported by the Netherlands Organization of Scientific Research (NWO-Vidi grant 16588, awarded to TvdH). VR was funded by NWO-Veni grant VI.Veni.212.059.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lammers, C., van de Ven, C.N., van der Heide, T. et al. Are Ecosystem Engineering Traits Fixed or Flexible: A Study on Clonal Expansion Strategies in Co-occurring Dune Grasses. Ecosystems 26, 1195–1208 (2023). https://doi.org/10.1007/s10021-023-00826-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00826-4