Abstract
Climate change modulates cold-marginal forest ecosystems through changing growth constraints. Understanding spatiotemporal variations in climate–growth relationships is essential to project forest ecosystem dynamics, and climate–environmental feedbacks. We explored variations in growth and climate–growth relationships, along the Arctic margin in north-western Europe, using Scots pine radial growth chronologies, climate data and links between the geographical origin of dominant air masses and growth-controlling variables. Analyses covered nineteenth century to early twenty-first century, with emphasis on two separate warming periods (early twentieth century, and late twentieth to early twenty-first century) and the intervening cooling period. The analyses revealed spatiotemporally unstable growth responses to climate along the Arctic margin. Spatial growth patterns were most similar during the cooling period. However, climate trends (warming, cooling) were weak drivers of growth-limiting climate variables. Instead, a transition in growth-limiting variables occurred throughout the analysed period. A wide range of growing season and non-growing season climate variables limited growth during the early twentieth century. Thereafter the number of growth-limiting variables progressively decreased. This change was accompanied by a contraction in the spatial correspondence between growth and climate, and by a shift in the geographical origin of dominant air masses. This was particularly emphasized close to the Atlantic during recent warming period. The weak association between growth-limiting variables and climate trends question projections of future ecosystem dynamics based on climate variables identified during specific periods (for example, recent warming period). Such projections may be misleading as the diversity of climate conditions constraining cold-marginal forests will be underestimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Growth responses to climate are spatiotemporally unstable along the Arctic margin
-
Climate trends are weak drivers of growth-limiting climate variables
-
Growth projections based on time-specific climate variables has to be questioned
Introduction
Tree growth at high latitudes is expected to sensitively track climate change, including concomitant change in the physiognomic structure of forests at the northern margin of the boreal zone (Briffa and others 1992; D’Arrigo and others 2006; Ohse and others 2012; Juday and others 2015; Hellmann and others 2016). Changes in the structure of cold-marginal forests are linked to complex interactions between both gradual and episodic abiotic and biotic processes (Hofgaard 1997; Holtmeier and Broll 2005; Hofgaard and others 2012; Scheffer and others 2012), with climate, and in particular summer temperature, put forward as a major driver (Briffa and others 1992; Szeicz and MacDonald 1995; Hofgaard and others 1999; Macias and others 2004; Porter and Pisaric 2011). Changing climate modulates ecosystem structure of cold-marginal forests through changing growth constraints and competitive relationships among the constituent tree species (Kellomäki and others 2008) with direct or indirect consequences to ecological diversity (Wielgolaski and others 2017).
The strength of tree growth response to summer temperature may vary considerably through time and space along with changes in climate trends, including non-growing season climate (Hofgaard and others 1999; Solberg and others 2002; Macias and others 2004; Porter and Pisaric 2011; Ols and others 2016, 2017). Climatic variability along geographical gradients may add to the spatiotemporal variation of climate–growth relationships (Hofgaard and others 1999; Huang and others 2010; Hellmann and others 2016; Matías and others 2017). Importantly, non-growing season conditions such as occurrence, timing and duration of snow cover may in a cascading way exercise different degrees of control upon tree growth along geographical gradients (Vaganov and others 1999; Sturm and others 2001; Räisänen 2008; Fréchette and others 2011). For example, snow accumulation may through its insulation effect prevent the development of growth-restricting low soil temperatures in cold regions, and contribute to growth-promoting meltwater during early-growing season in dry regions while delaying the start of the growing season in regions with surplus accumulation (Vaganov and others 1999; Sturm and others 2001; Räisänen 2008; Fréchette and others 2011). Despite recent increase in annual temperatures and growing season length at high latitudes (Karlsen and others 2009; IPCC 2014), no consistent increase in tree growth has been observed (D’Arrigo and others 2008; Porter and Pisaric 2011). The lack of general pattern may emerge from heterogeneous climate–growth relationships along geographical gradients (Huang and others 2010; Shuman and others 2011; Berner and others 2013; Hellmann and others 2016; Matías and others 2017).
Understanding climate–growth relationships is essential in the context of warming-induced changes in forest productivity and forest density, and concomitant changes in ecological conditions (Callaghan and others 2002; ACIA 2005; Parmesan 2006; Kaplan and New 2006; IPCC 2014; Juday and others 2015). In particular, enhanced growth of cold-marginal forests may affect the biological diversity inhabiting or seasonally depending on these forests (Callaghan and others 2002; Hofgaard and others 2012), and result in positive feedback to atmospheric warming through changes in albedo (Bright and others 2014; de Wit and others 2014). Climate-driven variability in forest growth will thus contribute to environmental changes at abiotic and biotic levels (Overpeck and others 1990; Gauthier and others 2015; Kuuluvainen and others 2017). Prolonged cold periods or cold events may cause growth disruptions and forest senescence independent of forest age, while prolonged warmer and otherwise favourable periods may increase forest vitality (Hofgaard 1997; Kullman 1998). This is exemplified for north-western Europe where cold-marginal forests, that were in a mangled physiognomic state at the end of the cold Little Ice Age period (Kullman 1987, 2005; Bradshaw and Zackrisson 1990), gained in vigour and growth during the early twentieth century warming (Kullman 1998). This shift in climate trend caused alterations of terrestrial ecosystems and recovery of profoundly stressed forest ecosystems (Hofgaard and others 1991; Overpeck and others 1997; Kullman 2005). In addition, cold episodes in the late 1980s caused growth reductions, and canopy and stand dieback due to a late thawing of ground frost and evolution of ground frost prevailing between cold seasons (Kullman 1991; Kullman and Högberg 1989). The ecological role and physiognomic structure of cold-marginal forests could thus, hypothetically and in a spatiotemporal manner, be controlled by altered climate–growth conditions at a scale from sub-optimal to optimal (Botkin and Nisbet 1992; Hofgaard 1997; Scheffer and others 2012).
North-western Europe is an ideal area for analyses of spatiotemporal climate–growth pattern due to its wide range of bioclimatic conditions within relatively short geographical distances (Moen 1999). This apparent bioclimatic diversity is caused by the proximity to the North Atlantic in the west, to the Barents Sea in the north, to continental areas in the south-east, and by orographic and topographic effects. The weather in the area is generally driven by air masses of Atlantic, Arctic and Russian origin, with the frequency of each air mass type changing at year, decade and longer timescales (Marshall and others 2016; Kivinen and others 2017; Ogurtsov and others 2017). Since the termination of the Little Ice Age in late nineteenth century (Grove 1988; Gates 1993) air mass circulation dynamics in the Arctic and sub-Arctic region, including north-western Europe, have triggered apparent, but not always spatiotemporally consistent, warming and cooling periods (Bradley and Jones 1993; ACIA 2005; Büntgen and others 2015; Marshall and others 2016; Kivinen and others 2017). Air mass circulation dynamics indirectly influence tree growth through their impact on local temperature and moisture (Bryson 1966). Hypothetically, these dynamics could therefore induce changes in growth-controlling climate variables. Knowledge of spatiotemporal changes in stationary vs. non-stationary climate–growth relationships is crucial, but not well understood, to predict growth, dynamics, and the ecological role of cold-marginal forest in a future climate (Gauthier and others 2015; Kuuluvainen and others 2017).
In this study, we explore the connection between growth dynamics of cold-marginal forests and climate trends along the Arctic margin in north-western Europe. We analyse spatiotemporal variation in tree growth patterns, using tree-ring width chronologies from Scots pine (Pinus sylvestris L.). The study area encompasses marked west-to-east and coast-to-inland bioclimatic gradients under the influence of Atlantic, Arctic and Russian air masses. The study covers the early nineteenth century to the early twenty-first century period and gives special emphasis to climate–growth relationships during two separate warming periods (in the early twentieth century, and in the late twentieth to early twenty-first century) and an intervening cooling period. The study assesses (1) tree growth patterns since the late Little Ice Age; (2) changes in main growth-controlling climate variable(s) in respect to both growing and non-growing season since the early twentieth century; (3) variations in the strength of climate–growth relationships between the two warming periods, between warming and cooling periods, and within specific warming and cooling periods; and (4) changes in the spatial extent and location of the spatial correspondence between growth and climate variables in relation to the geographical origin of dominant air masses.
Materials and Methods
Study Area and Sites
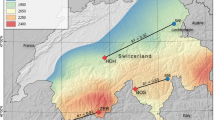
This study uses a dataset from the Norwegian Institute for Nature Research, consisting of adult Scots pine radial growth series collected along a 670-km longitudinal section of the northernmost European boreal forest margin, i.e. northern Norway and western Kola Peninsula in Russia (Figure 1). The area is characterized by coniferous forests (mainly Scots pine), birch forests, mixed pine and birch forests, and open tundra. Although the natural dynamics and distribution of these cold-marginal forests are primarily determined by climate, herbivores may locally play an important role (Stöcklin and Körner 1999; Kuuluvainen and others 2017). However, the impact by herbivores on adult Scots pine is limited. In addition, snow distribution and quality (for example, density and water content) are important factors regulating growth and stand dynamics of Scots pine at its northernmost locations (Stöcklin and Körner 1999).
Location of study sites (W1, W2, C1, C2, E1, E2; filled circles) and meteorological stations (stars) in northern Norway and north-western Russia (see Table 1 for site names, characteristics and data sources). All study sites are located along the northernmost range margin of Scots pine. Meteorological stations are indicated by the first two letters of their names. Grey shading indicates forest (boreal coniferous, mixed coniferous-broadleaved, and broadleaved forests). Arc-formed lines in the upper right-hand map indicate the origin of dominating air mass types affecting the region; Atlantic (AT), Arctic (AR) and Russian (RU), respectively. The lower part of the figure shows temperature and precipitation data for the meteorological stations representing each study site. Coastal station data are shown with black lines and inland station data with grey lines. The curves are based on the 1961–1990 normal period for all stations except Yukspor (Yu) for which only the Russian normal period 1881–1980 was available.
Selected study sites (selection design is detailed below) were all located along the Arctic margin, and included the world’s northernmost forest stand of Scots pine (Hofgaard and others 2013). All sites were characterized by open woodlands of scattered Scots pine trees in a sparse matrix of Mountain birch (Betula pubescens ssp. tortuosa (Ledeb.) Nyman) and situated on gentle south or south-west facing slopes with mesic soil conditions without permafrost (permafrost is only found in high-alpine areas or in peatlands). Dwarf shrubs (for example, Vaccinium spp., Empetrum sp.) dominated the field layer at all sites. The sites had no signs of recent logging or of recent or historic forest fire.
The climate of the study area is characterized by moist Atlantic air masses gradually replaced from west to east by dry Arctic air masses (Figure 1). Climate in the east is also characterized by air masses originating from the White Sea area in Russia. Three study regions were selected along this west–east climatic gradient: western Troms County in northern Norway (henceforward West; W), central northern Norway (Central; C) and easternmost northern Norway together with central Kola Peninsula (jointly named East; E). In each region, two sites representing coastal and inland conditions were selected, respectively. The study thus includes three coastal sites (termed 1; W1, C1 and E1) and three inland sites (termed 2; W2, C2 and E2) sampled along the main west–east climatic gradient (Figure 1; Table 1).
Northern coastal climate is generally characterized by moist and mild winters, and cool summers, while inland climate is characterized by cold and relatively dry winters and warm summers. This pattern is emphasized in the western region. The eastern region deviates from the general coast–inland pattern due to orographic effects, causing both wetter and cooler summers at the inland site (E2) than at the coast (Figure 1; Table S1).
The warmest month at all study sites is July and the coldest month is January, except at E2 where February is the coldest (Table S1). All sites are normally snow-covered from October to May. Since the termination of the Little Ice Age in the late nineteenth century (Grove 1988; Gates 1993), the study area has generally experienced both an increase in temperature, composed of two warming periods intervened by a cooling period, and an increase in annual total precipitation (ACIA 2005; Hanssen-Bauer and others 2015; Figures S1, S2). The first warming period occurred from the late nineteenth century to the 1930s and the second period from the late 1980s into the twenty-first century. The intervening cooling period was characterized by decreasing annual and seasonal temperatures, particularly during winter (Hanssen-Bauer and Førland 2000). At E2, the century-scale warming has been insignificant at annual level (Mathisen and others 2014), but significant for some spring and autumn months (Marshall and others 2016).
Sampling and Sample Preparation
A minimum of 25 healthy (that is, without visible crown or stem damage) adult Scots pine trees were sampled per site during summers 2007 and 2008. Two cores were taken from each tree at breast height and in opposite directions. Cores were mounted, dried and brought to the laboratory where they were planed with a scalpel. Zinc ointment was applied when needed to increase contrast between tree rings. Ring widths were measured with an accuracy of 0.001 mm using a sliding table system (LINTAB (Rinntech Inc., St Charles, IL, USA) for W and C cores, and Velmex (Velmex Inc. Bloomfield, NY, USA) for E cores), and a dissecting microscope with a magnification of 5–40×. The cores showed no growth ring pattern that could be interpreted as fire disturbance events.
Ring-width series of individual cores were cross-dated visually using pointer years (compare Figure 2) and statistically using COFECHA 6.06P and TSAP-Win (Holmes 1983; Rinn 2011). Series showing low correlation (r < 0.5) with the mean of all other series within a site were excluded from further analyses. This protocol resulted in a total of 343 series (out of 354), with 50–61 series per site (Table 1).
Radial growth patterns over the 1800–2006 period. Growth patterns are computed using a 10-year running average of site-specific low-pass chronologies. Horizontal thin dashed lines indicate the number of cores included in each chronology (right-hand y-axis). Vertical broken lines indicate shifts in climate trends (see Methods for description). Horizontal thick lines indicate visually defined periods with low decadal growth variability. Dots indicate selected negative pointer year (from left to right: 1837, 1868 (in C2 1866–1868 were all similarly narrow; 1867 is shown by open dot), 1903, 1910 and 1963). Grey shading indicates standard deviation of the annual average. See Table 1 for site and chronology characteristics and Figure 1 for site locations.
Chronology Construction and Dendrochronological Analyses
Two sets of site-specific chronologies were built using the ARSTAN program (Holmes 1992). The first set was used to analyse decadal to century-scale growth patterns, i.e. low-frequency signals, across bioclimatic gradients of the study area. To construct this first set, individual tree series were first power-transformed to stabilize variance (Cook and Peters 1997), and then detrended to remove age-related growth trends by fitting either a negative exponential or a negative slope linear model (Fritts 1976). Ring-width indices were computed as residuals from detrending. Residual series were finally averaged by robust mean to produce six site-specific low-pass chronologies.
The second set of chronologies was designed to analyse climate–growth relationships. As high-frequency growth variability better correlates with annual climate (Cook and Peters 1997), low-frequency signals were removed from each individual tree series using a 32-year spline. Ring-width indices were computed as residuals from detrending. Autocorrelation was then removed from residual series using autoregressive modelling (Cook and Peters 1997). All series were finally averaged at site level by robust mean to produce six site-specific high-pass chronologies. Mean sensitivity (Briffa and Jones 1990), expressed population signal and signal-to-noise ratio (Wigley and others 1984) were computed for all chronologies (low pass and high pass) for the 1901–2006 period (see below for period selection).
Correlation between high-pass chronologies was analysed using bootstrapped Pearson’s correlation (DendroClim 2002 program; Biondi and Waikul 2004) and principal component analyses (PCA). Chronologies were truncated to their common period 1800–2006 (Table 1). This period was further divided into four shorter periods (1800–1869, 1870–1939, 1940–1984 and 1985–2006). The length and time of division between these shorter periods were defined by shifts in long-term climate trends (Figure S1). The 1800–1869 period corresponds to the late Little Ice Age, the 1870–1939 period to the termination of the Little Ice Age and the first post Little Ice Age warming period, the 1940–1984 period to the mid-twentieth century cooling period, and the 1985–2006 period corresponds to the recent warming period.
Climate—Growth Analyses
As the distance between nearest climate stations and sampling sites varied among sites (Table 1), gridded climate data (CRU TS 3.22; Harris and others 2014) was used for climate–growth analyses. Site-specific gridded climate data for the 1901–2006 period (that is, common period between climate data and radial growth data) were retrieved from Climate Explorer (Trouet and Van Oldenborgh 2013, http://climexp.knmi.nl) using a 0.5° × 1° (lat. × long) grid. The 0.5° × 1° format was used to compensate for the spatial distortion in the curvature of latitude and longitude lines at high latitudes and obtain square-like site-specific climate cells. Correlations between station data and grid data are given in Table S1.
Relationships between high-pass chronologies (dependent variable) and monthly mean temperature and monthly total precipitation (predictors) were examined by correlation and response function analyses. Monthly climate predictors spanned from previous year June (Junet−1) to current year August (Augustt). Analyses were computed for the 1901–2006 period and for the herein three warming and cooling periods (1901–1939, 1940–1984 and 1985–2006). These analyses revealed numerous significant relationships with temperature, but only few with precipitation. To test for possible remaining tree-age effect on climate–growth relationships, tree series were split into old-tree and young-tree series on the basis of median tree age in year 1902 at each site. An old-tree and young-tree site-specific high-pass chronology were built and their responses to climate were compared. This test was run for the first warming period. To explore the significant relationships observed with monthly temperature at finer temporal scale additional moving correlation coefficients were computed over 1901–2006 using 20-year windows shifted 1 year at a time. The significance of coefficients (P < 0.05) was estimated by bootstrapping. Analyses were performed using the bootRes package in R (Zang and Biondi 2013). As analyses included the effects of previous year monthly predictors on current year growth, the first result year was 1902.
Local weather in our study area is strongly influenced by atmospheric dynamics (Figure S3), in particularly through the alternating dominance of air masses of Atlantic, Arctic and Russian origin. Air masses are characterized by spatially homogeneous temperature and precipitation properties. The spatial localization and extent of a significant climate–growth relationship is a good indicator of climate homogeneity through space, and therefore of air mass origin (Fritts 1991). To examine the link between temperature–growth relationships and air mass circulation dynamics, we computed spatial correlations between high-pass chronologies and monthly (from Junet−1 to Augustt) temperature fields (precipitation gave no interpretable results). To disclose time-dependent patterns the analyses were performed over the defined warming and cooling periods, and over 20-year intervals shifted 10 years at a time over the full 1901–2006 period. Analyses were performed online using Climate Explorer (Trouet and Van Oldenborgh 2013; http://climexp.knmi.nl) using a 0.5° × 0.5° (lat. × long.) grid. The 0.5° × 1° format allowing for squared-like climate cells at high latitudes was not available for online analyses.
Results
Chronology Statistics
Raw chronologies presented similar statistics across all sites. Mean series length was > 160 years for all chronologies (Table 1). All chronologies, except W2 and C1, had more than ten radii for the entire analysed 1800–2006 period (W2 from 1801 and C1 from 1811). Mean annual ring width was less than 1 mm for all chronologies (Table 1). All low- and high-pass chronologies had strong population signals, as indicated by their considerable variance in the first eigenvector, strong signal-to-noise ratios, and high expressed population signal (Table 1).
Long-Term Growth Patterns
Low-pass chronologies showed broadly congruent growth patterns (variations and trends) over the last two centuries, and presented common pointer years (Figure 2). However, different radial growth patterns were distinguished for the four climatically defined periods (Figure 2). The first period (late Little Ice Age 1800–1869) was characterized by concurring undulating growth pattern emphasized in the east. In the second period (warming 1870–1939) the undulating pattern characterizing the first period weakened, and disappeared completely at E2. Other sites, particularly C1, C2 and E1, showed relatively congruent growth patterns characterized by a marked growth decrease prior to 1910. In the third period (cooling 1940–1984) growth pattern similarity among sites was low and the undulating growth pattern reappeared at E2 (Figure 2). During the fourth period (warming 1985–2006), eastern and inland sites showed increased growth, while coastal sites towards the west showed reduced growth. Further, a weak or absent undulating growth pattern similar to the one observed at E2 during the second period, was a noticeable feature in the west and at C2 and E1 during large parts of the third and fourth period (Figure 2).
Growth Correlation Between Sites Through Time
Growth correlation between sites generally decreased with geographical distance, and its strength changed through time (Table 2). Although growth at coastal sites was mainly correlated with growth at their respective closest inland site, growth at inland sites tended to be most correlated with other inland sites. Weak coast–inland gradients (that is, high inter-site growth correlation) were noticeable in the west during the late Little Ice Age period, in the central region during the first warming period, and in the eastern region during both the cooling period and recent warming period (Table 2). Strong coast–inland gradients were shown in the west during the cooling period, in the central region during the late Little Ice Age period, and in the east during the first warming period. Growth correlations between sites generally decreased over time in the western region (strongest in the 1870–1939 period) and increased in the eastern region.
Temporal changes in gradient strength (that is, weak vs. strong gradients) included distinct variations in environmental distance between sites over the four periods (Figure 3) and sudden declines in inter-site growth correlations (Figure S4). The PCA revealed a fairly constant maximum environmental distance between site positions along the two first axes (PC1 and PC2) for all time periods, although individual site positions changed through time. PC1 and PC2 explained, respectively, 63.2–69.8% and 12.3–17.8% of the variance for the four analysed periods (Table S2). PC1 generally corresponded to an oceanic-continental gradient and PC2 to a west–east gradient. Other PCs had low explanatory power (Table S2). During the late Little Ice Age period, sites were aligned along a weak west–east gradient (PC2), with E2 being separated from other sites (Figure 3). During the first warming period, all sites except E2 were tightly clustered. During the cooling period, sites diverged and the west–east gradient was re-established, and W1 dissociated from other sites along both PC1 and PC2. During the recent warming period, site divergence progressed further, with all sites at more or less equal distance from each other along PC2. This recent west–east gradient also included a decrease in explained variance by PC1 and increase by PC2 (Table S2).
Temporal changes in environmental distance between sites shown as principal component score positions (PC1 and PC2) for the six site chronologies and selected periods. See Figure 1 and Table 1 for site locations and chronology characteristics, respectively, and Figure 2 for period selection. Note that inter-site environmental distance along PC2 changes through time although the maximum distance is more or less constant.
Climate–Growth Relationships over the 1901–2006 Period
The main climate–growth association for all sites over the 1901–2006 period, was a growth-promoting effect of Julyt and Augustt temperatures (Figure 4A; not significant for W2). The positive effect was particularly emphasized for Julyt and accompanied by negative correlation with Julyt precipitation (Figure 4A; for response function values see Figure S5). In addition to these common climate–growth associations, some site-specific associations with both growing and non-growing season factors were evident. Early current growing season temperature (Junet) had generally a low impact on radial growth (Figures 4, S5), but positively and significantly affected radial growth at W1 and E1 (Figure 4A). A strong and significant negative effect of Februaryt temperature was observed at W1 and C1 (Figure 4A). Additional significant, but weaker, positive associations were identified with Decembert−1 at W2 and C1, and Januaryt at E1. Regarding previous growing season, a positive effect of Junet−1 temperature at central sites, and a negative effect of Julyt−1 in the west were observed.
Associations between mean temperature (left-hand panels) and total precipitation (right-hand panels), and tree growth, presented as correlation between site-specific high-pass chronologies (indicated with different colours) and monthly CRU TS 3.22 climate grid data (Harris and others 2014) extracted using Climate Explorer (http://climexp.knmi.nl) and 0.5° lat. × 1° long. grid cells. Correlation coefficients are shown for 15 monthly variables spanning from June the year prior to growth to August the year of growth and for four periods A 1902–2006, B 1902–1939, C 1940–1984 and D 1985–2006 (compare Figure 2 for period selection). Error bars are shown for statistically significant values (P < 0.05). Growing season months are displayed in bold italic along the x-axes (Color figure online).
Climate–Growth Associations During Warming and Cooling Periods
The area-wide positive associations with current growing season (Julyt and Augustt) temperatures identified for the 1901–2006 period (Figure 4A) was also observed during the two warming periods. However, during the intervening cooling period, the positive effect of Augustt was replaced by a positive effect of Junet (Figure 4B–D). This growth-promoting effect of Junet during the cooling period was lost during the recent warming period, and instead became negative (significant negative influence for central sites; Figure 4D). Associations with non-growing season temperature were generally positive during the first warming period (early winter for E1 and E2, and late winter/spring for W1, W2, C2 and E1), and mixed (positive or negative) during the cooling period (for example, a negative association for February and a positive association for March at western and central sites). During the second warming period only one significant association (negative) with late winter/spring (April) was observed in the west (Figure 4B–D). Associations with previous growing season temperatures were only identified for the cooling period and the second warming period, but not for the first warming period. During the cooling period a negative impact of Julyt−1 was observed for all sites (not significant for W1 and C1), and this impact stayed negative for western and central cites during the second warming period. During this last period, an additional significant positive association with Junet−1 appeared at W and C sites (not significant at W1). Site-specific analyses run separately for old-tree and young-tree high-pass chronologies over the first warming period showed very similar climate–growth relationships (Figure S6).
The area-wide negative association for Julyt precipitation identified for the 1901–2006 period (Figure 4A) was only observed during the first warming period (Figure 4B), except at W1 where it prevailed throughout both warming periods and the cooling period (Figure 4B–D). Some significant associations with non-growing season precipitation were observed, but these associations differed across sites and between warming and cooling periods. Response function analyses revealed that these few significant associations were generally weak and inconsistent (Figures 4, S5). The strongest association with precipitation was a positive impact of increased January precipitation at C sites during the cooling period (Figures 4, S5).
The 20-year mowing window analyses (only computed for monthly temperature predictors; see Methods), further disclosed site-specific shifts in growth responses to temperature (both in the sign and trend of correlation values) between warming periods, and between warming and cooling periods (Figure 5A). However, site- and region-specific correlation values for individual months (see Figure S7) displayed apparent (Figure 5A, B) to very limited differences between climate periods (Figure 5A, C). The first warming period was characterized by shifts in correlation signs for most previous growing season and non-growing season months, and by stable correlations with Aprilt–Augustt (Figure 5A). During the cooling period, trends in correlation values with previous growing season and non-growing season months were reversed in comparison with those observed for the preceding warming period, and correlations with Februaryt–Mayt decreased to become negative. During the second warming period, correlation values generally stabilized at levels reached at the end of the cooling period, except for Februaryt and Junet.
Temporal variations in sign of the correlation coefficient between high-pass chronologies and CRU TS 3.22 monthly climate grid data (Harris and others 2014) extracted using Climate Explorer (http://climexp.knmi.nl) and 0.5° lat. × 1° long. grid cells. Variations are both shown as general pattern for all sites taken together (A) and for two selected months (B, C) (site-level data for all individual months is given in Figure S7). A Climate data includes monthly mean temperature from 1901 (first display year is 1902) to 2006 for 15 months spanning from June the year prior to growth (lowercase letters) to August the year of growth (uppercase letters). Correlation sign and strength are shown by colour according to the scale below the x-axis. Arrows indicate trends towards more positive or less positive correlation, respectively. The number of arrows indicates the duration of such trend (short correlation change events are only shown with colour intensity changes). Correlation coefficients are computed for 20-year moving windows shifted 1 year at a time (middle year plotted). B, C Site-level correlations for two selected individual months (calculated as described for (A), current year March and July, respectively. The site legend presented in C accounts for both B and C, and bold lines indicate significant correlations (95% bootstrapping confidence interval).
Spatial Extent of Climate–Growth Associations Through Time
Spatial analyses broadly identified Julyt temperature as the main growth-controlling variable during both warming/cooling periods and sequential 20-y intervals (Figures 6, 7). However, these analyses revealed clear temporal differences in the spatial location and extent of this positive association with Julyt temperature across the three warming/cooling periods (Figure 6) and the finer 20-y interval scale (Figure 7).
Significant spatial correlations (P < 0.05) between current year July temperature and high-pass chronologies over warming and cooling periods since the beginning of the twentieth century (1901–2006) (compare Figure 2 for period selection). Significant spatial correlations (P < 0.05) for other months are given in Table S3. Monthly temperatures consisted of CRU TS 3.22 temperature grid data (Harris and others 2014) at a 0.5° lat. × 0.5° long. resolution available from Climate Explorer (http://climexp.knmi.nl). See Methods for further explanations.
Significant spatial-field correlations (P < 0.05) between current year July temperature and high-pass chronologies (maps). The analyses cover sequential 20-year intervals with 10-year overlap for the 1901–2006 period. Periods with no significant association with Julyt temperature, but showing significant associations with other monthly temperature predictors are displayed with symbols (details are given in Table S4): open circles for mixed association including both non-growing season and growing season months; filled half-circles for previous growing season months; squared half-circles for non-growing season months; and (none) for no significant correlation with any month. Position of displayed July correlations and half-circles indicates location of dominating air mass association. Monthly temperatures consisted of CRU TS 3.22 temperature grid data (Harris and others 2014.) available from Climate Explorer (http://climexp.knmi.nl) at a 0.5° lat. × 0.5° long. resolution. See Methods for further explanations.
During the first warming and the cooling period, the positive association with Julyt temperature was characterized by a wide spatial extent located towards Atlantic and Arctic sectors (emphasized at E1), with increased Arctic importance at eastern sites (Figure 6). During the second warming period this wide positive association with Julyt weakened at all sites except for E2 where the Russian sector dominated the association. During this last period, the coastal sites W1 and C1 became negatively associated with southern temperature conditions (Figure 6). Sites with low positive or negative association with Julyt temperature during this last period were characterized by significant spatial correlations with spring or early summer factors (Table S3).
Twenty-year interval analyses revealed a frequent disappearance of the significant spatial association with Julyt temperature at western and central sites (in particular W1) (Figure 7). Intervals during which this association disappeared were instead characterized by either significant spatial associations with non-growing season temperature (during early and late twentieth century intervals) or with previous growing season temperature (during mid-century intervals) (Figure 7; Table S4). The only site with a continuous (that is, for all 20-y intervals) spatial association with Julyt temperature was E1, although the spatial extent of the association changed through time.
Discussion
Despite recent warming trend (IPCC 2014), our study provides no strong evidence of decreased sensitivity to summer (JJA) temperature over recent decades, as commonly reported earlier (Jacoby and D’Arrigo 1995; Briffa and others 1998; Galván and others 2015). Instead, we observed a continuous significant association between tree growth and main summer month (July) climate for most sites over the entire twentieth century. However, our results highlighted shifts in both the number and the type of growth-limiting climate variables, throughout the studied period. In particular, although growth was significantly associated with a number of non-growing season climate variables during most of the twentieth century, these associations weakened during the recent warming period. Even though this weakening may appear associated with the shift from the cooling period to the recent warming period, our study showed no specific set of growth-limiting climate variables for either period. Instead, tree growth has become increasingly controlled by growing season variables since early twentieth century. Hypothetically, this relates to a long-term post Little Ice Age transition from sub-optimal (growth restricting) to optimal (non-growth restricting) non-growing season climate, while main growing season month(s) generally stayed sub-optimal.
Shifts between sub-optimal and optimal climate conditions affect tree growth and alter its responses to climate. In more southern boreal areas, an optimal situation might have been reached for most monthly climate variables, explaining the recently observed divergence between growing-season climate and growth (Jacoby and D’Arrigo 1995; Briffa and others 1998; Galván and others 2015). This reduction or loss of correlation with growing season climate variables has been interpreted as a sign of loss of sensitivity to climate (Briffa and others 1998; D’Arrigo and others 2008) and weakened ability of northern boreal ecosystems to adapt to ongoing climate change, both from a quantitative (rate) and qualitative (parameters) perspective (IPCC 2014). Divergences from previous climate–growth associations may instead, as shown in our study, indicate changes in growth-constraining climate. The duration, frequency and magnitude of such changes will consequently vary in a spatiotemporal manner (Charney and others 2016; Hellmann and others 2016). Accordingly, shifts in climate–growth associations may be linked to changes in growth-constraining climate (quantitatively and qualitatively) where non-growing season vs. growing season processes are of major importance (Callaghan and others 2013).
Although a common climate trend is assumed to homogenize growth responses across spatial scales (Shestakova and others 2016), climate variability might mask trend-driven growth responses. This was exemplified for the two analysed warming periods. Despite similar range and rate in temperature increase (Figure S1), climate change promoted growth in most of the area during the early twentieth century warming (weak response in continental east where warming had been insignificant), but only at central continental and eastern sites during the recent warming period. This result questions the role of climate trends as a harmonizing factor of growth responses to climate in the studied area.
The recent weakening of non-growing season control on growth might, if warming continues, be an early indicator of future warming-induced negative impacts of rising non-growing season temperatures on tree growth processes. The recent growth decrease observed at the western coastal site supports this hypothesis, and suggests that growth at sites under strong influence of Atlantic air masses are currently more sensitive to ongoing climate change than sites under dominating influence of air masses of Arctic and eastern origin. Explaining the recent growth response to climate warming at western sites remains challenging. Hypothetically, the concurrent and significant precipitation increase across the study area might have passed a negative threshold in the west, and thus caused unfavourable growth conditions (Zhang and others 2013; Zeppel and others 2014), although significant correlations with precipitation variables are generally weak or limited (present study and Macias and others 2004). In the warm north-western coastal Norway, increased autumn and early winter precipitation (rain) may delay the build-up of an insulating snow cover, and thereby mediate development of colder soil conditions persisting into the start of the growing season (Vaganov and others 1999; Sturm and others 2001; Fréchette and others 2011). In addition, increased late winter and spring precipitation (snow) at high latitudes may delay the onset of the growing season (Vaganov and others 1999). In the colder eastern region, characterized by negligible temperature increase and longer a snow season, increased precipitation might promote growth as a result of better soil insulation (Fréchette and others 2011).
Although the positive association between tree growth and July temperature was consistent across sites and over time, its spatial location and extent suggested an important role of air mass origin in shaping this climate–growth association. Although this positive association was spatially wide and strong during the early warming and the cooling periods, it weakened and contracted over recent warming period. This was particular observed for western and central sites, where a negative association with air masses of southern origin emerged. The recent weakening and spatial contraction of the July temperature association might underline that climatic conditions in regions dominated by air masses of Atlantic origin might have become less favourable to tree growth compared to more easterly regions. The observed temporal and spatial instability in climate–growth associations questions the general applicability of the assumed uniformitarianism principle at geographical and temporal scales beyond the ones of performed studies (Hughes and others 2011; Hellmann and others 2016; Matías and others 2017). This lack of general applicability is also pointed out at ecosystem level, where correlations between climate and ecosystem dynamics at a large regional scale might hide actual causes of ecosystem dynamics (Callaghan and others 2013).
The relation between tree growth and forest dynamics at high latitudes is not straightforward (Holtmeier and Broll 2007; Hofgaard and others 2009). However, a better understanding of spatiotemporal variability in growth-limiting climate and climate–growth associations might improve the modelling of future productivity, growth and dynamics of cold-marginal forests. Recent studies have documented that the observed rates of forest latitudinal and altitudinal migration in sub-Arctic areas are lagging behind the modelled rates by at least one order of magnitude (Van Bogaert and others 2011; Hofgaard and others 2013; Mathisen and others 2014). We argue that, predictions of future cold-marginal ecosystem dynamics will likely be misleading if merely based on temperature variability of the warmest month (the most common variable used in studies estimating future dynamics of cold-marginal northern forests). Taking into account the spatiotemporal variation in growth responses and the inclusion of both growing season and non-growing season climate variables is essential to improve the reliability of model results (Matías and others 2017). The herein observed spatiotemporal variation in climate–growth associations could partly be responsible for reported mismatch between modelled and empirical estimates (Van Bogaert and others 2011; Hofgaard and others 2013).
Conclusion
Differences in forests’ responses to climate across space and time along the Arctic margin are of important ecological, climatic and socio-economical relevance (Callaghan and others 2002). A narrow focus on the strongest climate–growth association identified over long-term periods might produce misleading scenarios of ecosystem dynamics. Predictions of future forest productivity for terrestrial-climate feedback scenarios, management purposes, resource utilization planning or other purposes, have to account for spatiotemporal heterogeneous growth responses to climate. Knowledge bound in limited spatial or temporal scales cannot be interpolated to other spatiotemporal scales/frames without thoughtful considerations of non-conformities (Briffa and others 1998). Recorded long-term means of climate–growth associations might hide site-specific information of higher temporal resolution that are essential for scenario building.
References
ACIA. 2005. Arctic climate impact assessment. Cambridge: Cambridge University Press.
Berner LT, Beck PSA, Bunn AG, Goetz SJ. 2013. Plant response to climate change along the forest-tundra ecotone in northeastern Siberia. Global Change Biology 19:3449–62.
Biondi F, Waikul K. 2004. DENDROCLIM2002: AC++ program for statistical calibration of climate signals in tree-ring chronologies. Computers & Geoscience 30:303–11.
Botkin DB, Nisbet RA. 1992. Forest response to climate change: effects of parameter estimation and choice of weather patterns on the reliability of projections. Climate Change 20:87–211.
Bradley RS, Jones PD. 1993. ‘Little Ice Age’ summer temperature variations: their nature and relevance to recent global warming trends. The Holocene 3:367–76.
Bradshaw R, Zackrisson O. 1990. A two thousand year history of a northern Swedish boreal forest stand. Journal of Vegetation Ecology 1:519–28.
Briffa KR, Jones PD. 1990. Basic chronology statistics and assessment. In: Cook ER, Kairiukstis LA, Eds. Methods of dendrochronology: applications in the environmental science. Dordrecht: Kluwer Academic Publishers.
Briffa KR, Jones PD, Bartholin TS, Eckstein D, Schweingruber FH, Karlen W, Zetterberg P, Eronen M. 1992. Fennoscandian summers from AD 500: temperature changes on short and long timescales. Climate Dynamics 7:111–19.
Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Shiyatov SG, Vaganov EA. 1998. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 391:678–82.
Bright RM, Antón-Fernández C, Astrup R, Cherubini F, Kvalevåg M, Strømman A. 2014. Climate change implications of shifting forest management strategy in a boreal forest ecosystem of Norway. Global Change Biology 20:607–21.
Bryson RA. 1966. Air masses, streamlines, and the boreal forest. Geographical Bulletin 8:228–69.
Büntgen U, Hellmann L, Tegel W, Normand S, Myers-Smith I, Kirdyanov AV, Nievergelt D, Schweingruber FH. 2015. Temperature-induced recruitment pulses of Arctic dwarf shrub communities. Journal of Ecology 103:489–501.
Callaghan TV, Crawford RMM, Eronen M, Hofgaard A, Payette S, Rees WG, Skre O, Sveinbjörnsson B, Vlassova TK, Werkman BR. 2002. The dynamics of the tundra taiga boundary: an overview and a co-ordinated and integrated approach to research. Ambio Special Report 12:3–5.
Callaghan TV, Jonasson C, Thierfelder T, Yang Z, Hedenås H, Johansson M, Molau U, Van Bogaert R, Michelsen A, Olofsson J, Gwynn-Jones D, Bokhorst S, Phoenix G, Bjerke JW, Tømmervik H, Christensen TR, Hanna E, Koller EK, Sloan VL. 2013. Ecosystem change and stability over multiple decades in the Swedish subarctic: complex processes and multiple drivers. Philosophical Transactions of the Royal Society B 368:20120488.
Charney ND, Babst F, Poulter B, Record S, Trouet VM, Frank D, Enquist BJ, Evans MEK. 2016. Observed forest sensitivity to climate implies large changes in 21st century North American forest growth. Ecology Letters 19:1119–28.
Cook ER, Peters K. 1997. Calculating unbiased tree-ring indices for the study of climatic and environmental change. The Holocene 7:361–70.
D’Arrigo R, Wilson R, Jacoby G. 2006. On the long-term context for late twentieth century warming. Journal of Geophysical Research 111:D03103.
D’Arrigo R, Wilson R, Liepert B, Cherubini P. 2008. On the ‘Divergence Problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global and Planetary Change 60:289–305.
de Wit HA, Bryn A, Hofgaard A, Karstensen J, Kvalevåg M, Peters G. 2014. Climate warming feedback from mountain birch forest expansion: reduced albedo dominates carbon uptake. Global Change Biology 20:2344–55.
Fréchette E, Ensminger I, Bergeron Y, Gessler A, Berninger F. 2011. Will changes in root-zone temperature in boreal spring affect recovery of photosynthesis in Picea mariana and Populus tremuloides in a future climate? Tree Physiology 31:1204–16.
Fritts HC. 1976. Tree-rings and climate. London: Academic Press.
Fritts HC. 1991. Reconstructing large-scale climatic patterns from tree-ring data: t diagnostic analysis. Tucson: University of Arizona Press.
Galván JD, Büntgen U, Ginzler C, Grudd H, Gutiérrez E, Labuhn I, Camarero JJ. 2015. Drought-induced weakening of growth–temperature associations in high-elevation Iberian pines. Global and Planetary Change 124:95–106.
Gates DM. 1993. Climate change and its biological consequences. Sunderland: Sinauer Associates Inc.
Gauthier S, Bernier P, Kuuluvainen T, Shvidenko AZ, Schepaschenko DG. 2015. Boreal forest health and global change. Science 349:819–22.
Grove JM. 1988. The little ice age. London: Methuen.
Hanssen-Bauer I, Førland E. 2000. Temperature and precipitation variations in Norway 1900–1994 and their links to atmospheric circulation. International Journal of Climatology 20:1693–708.
Hanssen-Bauer I, Førland EJ, Haddeland I, Hisdal H, Mayer S, Nesje A, Nilsen JEØ, Sandven S, Sandø AB, Sorteberg A, Ådlandsvik B. 2015. Klima i Norge 2100. Kunnskapsgrunnlag for klimatilpasning oppdatert 2015. Norsk klimaservicesenter rapport, no. 2/2015.
Harris I, Jones PD, Osborn TJ, Lister DH. 2014. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 dataset. International Journal of Climatology 34:623–42.
Hellmann L, Agafonov L, Ljungqvist FC, Churakova O, Düthorn E, Esper J, Hülsman L, Kirdyanov AV, Moiseev P, Myglan VS, Nikolaev AN, Reinig F, Schweingruber FH, Solomina O, Tegel W, Büntgen U. 2016. Diverse growth trends and climate responses across Eurasia’s boreal forest. Environmental Research Letters 11:074021.
Hofgaard A. 1997. Structural changes in the forest-tundra ecotone: a dynamic process. Huntley B, Cramer W, Morgan AV, Prentice HC, Allen JRM, Eds. Past and future rapid environmental changes: the spatial and evolutionary responses of terrestrial biota. NATO ASI Series, Vol. I 47. Springer. pp 255–63.
Hofgaard A, Dalen L, Hytteborn H. 2009. Tree recruitment above the treeline and potential for climate driven treeline change. Journal of Vegetation Science 20:1133–44.
Hofgaard A, Harper KA, Golubeva E. 2012. The role of the circumarctic forest-tundra ecotone for arctic biodiversity. Biodiversity 13:174–81.
Hofgaard A, Kullman L, Alexandersson H. 1991. Response of old-growth montane Picea abies (L.) Karst. forest to climatic variability in northern Sweden. New Phytologist 119:585–94.
Hofgaard A, Tardif J, Bergeron Y. 1999. Dendroclimatic response of Picea mariana and Pinus banksiana along a latitudinal gradient in the eastern Canadian boreal forest. Canadian Journal of Forest Research 29:1333–46.
Hofgaard A, Tømmervik H, Rees G, Hanssen F. 2013. Latitudinal forest advance in northernmost Norway since the early 20th century. Journal of Biogeography 40:938–49.
Holmes RL. 1983. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin 43:69–78.
Holmes RL. 1992. Dendrochronology Program Library, Instruction and Program Manual. Tucson: Laboratory of Tree-Ring Research, University of Ari.
Holtmeier FK, Broll G. 2005. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography 14:395–410.
Holtmeier FK, Broll G. 2007. Treeline advance—driving processes and adverse factors. Landscape Online 1:1–33.
Huang JG, Tardif J, Bergeron Y, Denneler B, Berninger F, Girardin MP. 2010. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Global Change Biology 16:711–31.
Hughes MK, Swetnam TW, Diaz HF. Eds. 2011. Dendroclimatology: progress and prospects. Developments in Paleoenvironmental Research 11. Springer.
IPCC. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Pachauri RK, Meyer LA, Eds. Geneva, Switzerland: IPCC.
Jacoby GC, D’Arrigo RD. 1995. Tree ring width and density evidence of climatic and potential forest change in Alaska. Global Biogeochemical Cycles 9:227–34.
Juday GP, Alix C, Grant TAIII. 2015. Spatial coherence and change of opposite white spruce temperature sensitivities on floodplains in Alaska confirms early-stage boreal biome shift. Forest Ecology and Management 350:46–61.
Kaplan JO, New M. 2006. Arctic climate change with a 2°C global warming: timing, climate patterns and vegetation change. Climatic Change 79:213–41.
Karlsen SR, Elvebakk A, Høgda KA, Johansen B. 2006. Satellite-based mapping of the growing season and bioclimatic zones in Fennoscandia. Global Ecology and Biogeography 15:416–30.
Karlsen SR, Høgda KA, Wielgolaski FE, Tolvanen A, Tømmervik H, Poikolainen J, Kubin E. 2009. Growing-season trends in Fennoscandia 1982–2006, determined from satellite and phenology data. Climate Research 39:275–86.
Kellomäki S, Peltola H, Nuutinen T, Korhonen KT, Strandman H. 2008. Sensitivity of managed boreal forests in Finland to climate change, with implications for adaptive management. Philosophical Transactions of the Royal Society B 363:2341–51.
Kivinen S, Rasmus S, Jylhä K, Laapas M. 2017. Long-term climate trends and extreme events in northern Fennoscandia (1914–2013). Climate 5(1):16.
Kullman L. 1987. Little Ice Age decline of cold marginal Pinus sylvestris forest in the Swedish Scandes. New Phytologist 106:567–84.
Kullman L. 1991. Ground frost restrictions of subarctic Picea abies forest in northern Sweden. A dendroecological analyses. Geografiska Annaler 73A:167–78.
Kullman L. 1998. Tree-limits and montane forests in Swedish Scandes: sensitive biomonitors of climate change and variability. Ambio 27:312–21.
Kullman L. 2005. Pine (Pinus sylvestris) treeline dynamics during the past millennium—a population study in west-central Sweden. Annales Botanici Fennici 42:95–106.
Kullman L, Högberg N. 1989. Rapid natural decline of upper montane forests in the Swedish Scandes. Arctic 42:217–26.
Kuuluvainen T, Hofgaard A, Aakala T, Jonsson BG. 2017. North Fennoscandian mountain forests: history, composition, disturbance dynamics and the unpredictable future. Forest Ecology and Management 385:140–9.
Macias M, Timonen M, Kirchhefer AJ, Lindholm M, Eronen M, Gutiérrez E. 2004. Growth variability of Scots pine (Pinus sylvestris) along a West-East gradient across northern Fennoscandia: a dendroclimatic approach. Arctic, Antarctic, and Alpine Research 36:565–74.
Marshall GJ, Vignols RM, Rees WG. 2016. Climate change in the Kola Penninsula, Arctic Russia, during last 50 years from meteorological observations. Journal of Climate 29:6823–40.
Mathisen IE, Mikheeva A, Tutubalina OV, Aune S, Hofgaard A. 2014. Fifty years of tree line change in Khibiny Mountains, Russia: advantages of combined remote sensing and dendroecological approaches. Applied Vegetation Science 17:6–16.
Matías L, Linares JC, Sánchez-Miranda A, Jump AS. 2017. Contrasting growth forecasts across the geographical range of Scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Global Change Biology 23:4106–16.
Moen A. 1999. National atlas of Norway: vegetation. Hønefoss: Norwegian Mapping Authority.
Ogurtsov M, Lindholm M, Jalkanen R, Veretenenko SV. 2017. North Atlantic sea surface temperature, solar activity and the climate of Northern Fennoscandia. Advances in Space Research 59:980–6.
Ohse B, Jansen F, Wilmking M. 2012. Do limiting factors at Alaskan treelines shift with climatic regimes? Environmental Research Letters 7:015505.
Ols C, Girardin MP, Hofgaard A, Bergeron Y, Drobyshev I. 2017. Monitoring climate sensitivity shifts in tree-rings of eastern boreal North America using model-data comparison. Ecosystems. https://doi.org/10.1007/s10021-017-0203-3.
Ols C, Hofgaard A, Bergeron Y, Drobyshev I. 2016. Previous growing season climate controls the occurrence of black spruce growth anomalies in boreal forests of Eastern Canada. Canadian Journal of Forest Research 46:696–705.
Overpeck J, Hughen K, Hardy D, Bradley R, Case R, Douglas M, Finney B, Gajewski K, Jacoby G, Jennings A, Lamoureux S, Lasca A, MacDonald G, Moore J, Retelle M, Smith S, Wolfe A, Zielinski G. 1997. Arctic environmental change of the last four centuries. Science 278:1251–6.
Overpeck JT, Rind D, Goldberg R. 1990. Climate induced changes in forest distribution and vegetation. Nature 343:51–3.
Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics 37:637–69.
Porter TJ, Pisaric MFJ. 2011. Temperature-growth divergence in white spruce forests of Old Crow Flats, Yukon Territory, and adjacent regions of northwestern North America. Global Change Biology 17:3418–30.
Räisänen J. 2008. Warmer climate: less or more snow? Climate Dynamics 30:307–19.
Rinn F. 2011. TSAP-Win. Time Series Analysis and Presentation for Dendrochronology and Related Applications. Version 4.64 for Microsoft Windows. User Reference. Heidelberg, Germany: Rinntech.
Scheffer M, Hirota M, Holmgren M, Van Nes EH, Chapin FS. 2012. Thresholds for boreal biome transitions. Proceedings of the National Academy of Sciences USA 109:21384–9.
Shestakova TA, Gutiérrez E, Kirdyanov AV, Camarero JJ, Génova M, Knorre AA, Linares JC, Resco de Diosg V, Sánchez-Salguerof R, Voltas J. 2016. Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proceedings of the National Academy of Sciences 113:662–7.
Shuman JK, Shugart HH, O’Halloran TL. 2011. Sensitivity of Siberian larch forests to climate change. Global Change Biology 17:2370–84.
Solberg B, Hofgaard A, Hytteborn H. 2002. Shifts in radial growth responses of coastal Picea abies induced by climate change during the 20th century, Central Norway. Ecoscience 9:79–88.
Szeicz JM, MacDonald GM. 1995. Age-dependent tree-growth response of sub-arctic white spruce to climate. Canadian journal of Forest Research 24:120–32.
Stöcklin J, Körner C. 1999. Recruitment and mortality of Pinus sylvestris near the Nordic treeline: the role of climate change and herbivory. Ecological Bulletin 47:168–77.
Sturm M, McFadden JP, Liston GE, Chapin FSIII, Racine CH, Holmgren J. 2001. Snow–shrub interactions in arctic tundra: a hypothesis with climate implications. Journal of Climate 14:336–44.
Trouet V, Van Oldenborgh GJ. 2013. KNMI climate explorer: a web-based research tool for high-resolution paleoclimatology. Tree-Ring Research 69:3–13.
Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP. 1999. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400:149–51.
Van Bogaert R, Haneca K, Hoogesteger J, Jonasson C, De Papper M, Callaghan TV. 2011. A century of tree line changes in sub-arctic Sweden shows local and regional variability and only minor influence of 20th century climate warming. Journal of Biogeography 38:907–21.
Wielgolaski F, Hofgaard A, Holtmeier FK. 2017. Sensitivity to environmental change of the treeline ecotone and its associated biodiversity in European mountains. Climate Research 73:151–66.
Wigley TML, Briffa KR, Jones PD. 1984. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23:201–13.
Zang C, Biondi F. 2013. Dendroclimatic calibration in R: the bootRes package for response and correlation function analysis. Dendrochronologia 31:68–74.
Zeppel MJB, Wilks JV, Lewis JD. 2014. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences 11:3083–93.
Zhang Y, Moran MS, Nearing MA, Ponce Campos GE, Huete AR, Buda AR, Bosch DD, Gunter SA, Kitchen SG, McNab WH, Morgan JA, McClaran MP, Montoya DS, Peters DPC, Starks PJ. 2013. Extreme precipitation patterns and reductions of terrestrial ecosystem production across biomes. Journal of Geophysical Research 118:148–57.
Acknowledgements
This paper is a product under the IPY core project PPS Arctic (http://ppsarctic.nina.no) and was financially supported by the Research Council of Norway (RCN) through Grant No. 176065/S30 to AH. In addition, we are thankful for support from the Norwegian Institute for Nature Research core funding from RCN (Grant No. 160022/F40 to AH), ECOFUNC (RCN Grant No. 244557/RI part I to AH) and NordicDendro, a Nordic-Canadian network on forest growth research, supported by the Nordic Council of Ministers (Grant No. 12262 to ID). We thank Ingrid E. Mathisen, Sigrun Aune and Erin Sandberg for assistance in the field, Kari Sivertsen for help with Figures 1 and 7, and Mikhail Zimin and Yurate Plyushkyiavichyute for help with the Russian climate data. Finally, we thank two anonymous reviewers for their constructive comments on the initial manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hofgaard, A., Ols, C., Drobyshev, I. et al. Non-stationary Response of Tree Growth to Climate Trends Along the Arctic Margin. Ecosystems 22, 434–451 (2019). https://doi.org/10.1007/s10021-018-0279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0279-4