Abstract
Invasive nitrogen (N)-fixing plants often fundamentally change key ecosystem functions, particularly N-cycling. However, the consequences of this for litter decomposition, and the mechanisms that underpin ecosystem responses, remain poorly understood. Moreover, few studies have determined how nutrient pools and fluxes shift as invader density increases and whether these effects persist following invader removal, despite the importance of this for understanding the timing and magnitude of invader impacts in ecosystems. We tested how the decomposition rates of four co-occurring grass species were influenced by changes in the density of the globally invasive N-fixing shrub Cytisus scoparius L. (Scotch broom) and whether these effects persisted following invader removal. We used a series of laboratory decomposition assays to disentangle the roles of changes in both litter quality and soil properties associated with increases in broom density. Broom invasion created a soil environment, such as higher rates of net N-mineralisation, which retarded litter decomposition. Litter C/N ratios of co-occurring species decreased as broom density increased, yet this had no effect on decomposition rates. Most relationships between broom density and impacts were nonlinear; this could explain some of the reported variation in invasive species impacts across previous studies that do not account for invader density. Ecosystem properties only partially recovered following invader removal, as broom left a legacy of increased N-availability in both soils and litter. Our findings suggest that invasive N-fixer impacts on soil properties, such as N-availability, were more important than changes in litter quality in altering decomposition rates of co-occurring species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant invasions, particularly by nitrogen (N)-fixing species, are changing the global N-cycle through an increase in ecosystem N pools and fluxes worldwide (Liao and others 2008; Ehrenfeld 2010; Castro-Díez and others 2014). Native N-fixing plants are likely to have similar influences on ecosystem properties (St. John and others 2012); however, N-fixing species frequently constitute a much higher proportion of non-native floras than comparable native floras (Ehrenfeld 2003; Levine and others 2003; Peltzer and others 2016), suggesting invasive N-fixers may have larger impacts on global N pools and fluxes. These impacts are likely to have significant consequences for ecosystem processes such as litter decomposition (Liao and others 2008; Vilà and others 2011), which is a crucial part of biologically important nutrient cycles. Decomposition rates largely determine the availability of key nutrients for plant growth and consequently influence plant community composition, net primary production and biodiversity (Wardle and others 2004; Bardgett 2005). However, we still lack a mechanistic understanding of invasive N-fixer impacts on litter decomposition rates. Such understanding may help to explain the large variation in the direction and magnitude of invasive species impacts in ecosystems (Vilà and others 2011; Castro-Díez and others 2014). Further, the role of invasive species density in determining their impact is also poorly understood (Yokomizo and others 2009; Ehrenfeld 2010; Vilà and others 2011) because the majority of invader impact studies use end-point comparisons, which compare imprecisely defined “heavily” invaded sites with uninvaded sites (Ehrenfeld 2010; Jackson and others 2014). These knowledge gaps, along with a paucity of information on the persistence of impacts following invader removal (Von Holle and others 2013), are hindering effective management of invaded ecosystems (Hulme and others 2013).
The impacts of N-fixing invaders on decomposition rates are likely driven by several mechanisms. Firstly, invasive N-fixers often increase inorganic N concentrations and N-mineralisation rates in the soil (Liao and others 2008; Vilà and others 2011). Higher soil N-availability can lead to either increases or decreases in decomposition rates depending on other factors, such as litter quality (Hobbie 2000; Knorr and others 2005; Craine and others 2007). Secondly, increases in N-availability can alter soil microbial functioning, for example by reducing the fungal/bacterial ratio (de Vries and others 2006) and shifting bacterial community composition (Ramirez and others 2012; Leff and others 2015). Such changes in composition have been linked to both higher (Wardle and others 2004) and lower (Ramirez and others 2012) litter decomposition rates. Finally, invasive N-fixers can affect decomposition rates by altering litter quality. This occurs due to the higher N-content of their own litter (Liao and others 2008), but also due to an increase in the N-content of co-occurring species litter following increased soil N-availability (Xia and Wan 2008; Lu and others 2011). This effect may be stronger for co-occurring exotic species than co-occurring native species, due to greater trait plasticity (Davidson and others 2011; Peltzer and others 2016). Many studies focus on litter quality, particularly litter C/N ratio, as the most important driver of decomposition rates (for example Taylor and others 1989; Aerts 1997; Perez-Harguindeguy and others 2000), and increases in litter N-content are likely, but not certain, to increase decomposition rates (Perez-Harguindeguy and others 2000; Hobbie 2015). However, other drivers may also be important, particularly in the context of N-fixer invasions. To date, we have little knowledge of how the decomposition rates of co-occurring species change as invasive N-fixers increase in density, or of the mechanisms that drive these changes.
Management of invasive N-fixing species often involves removal of the invader, yet invader impacts on ecosystem properties and processes may persist following removal, resulting in a long-term legacy (Corbin and D’Antonio 2012). For example, soil N-availability can remain elevated for 14 years following removal of an invasive N-fixer (Von Holle and others 2013), but the consequences of this for many ecosystem processes such as decomposition are unresolved (Corbin and D’Antonio 2012). Legacy effects of invaders on decomposition rates are likely also driven by shifts in plant community composition that frequently occur following invader removal. For example, exotic nitrophilic grasses rather than native species often dominate areas where invasive woody plants, such as Scotch broom (Cytisus scoparius, L.) (Williams 1998; Grove and others 2015) and Pinus contorta (Dickie and others 2014), have been removed. The potentially higher trait plasticity of these exotics, compared to co-occurring native species (Davidson and others 2011), suggests they may respond to higher soil N-availability with a greater increase in litter N concentrations than natives (Peltzer and others 2016). The combination of both biological and biogeochemical legacy effects may ultimately result in permanently altered rates of decomposition and nutrient cycling, thereby giving rise to alternative stable states or “novel” ecosystems (Norton 2009). This could have negative implications for biodiversity and severely impede efforts to restore native ecosystems (Corbin and D’Antonio 2012).
Here, we aim to understand how the density of the widespread invasive N-fixer Scotch Broom (Cytisus scoparius L., hereafter broom) impacts soil properties, litter quality and decomposition rates of co-occurring native and exotic species in a New Zealand montane ecosystem and whether those impacts persist following broom removal. Broom is an effective N-fixer, deriving up to 81% of the N in its aboveground tissue from the atmosphere (Watt and others 2003). It is native to Europe, Central Asia and West Africa and is a widespread weed in New Zealand, the USA, Australia, Canada, Chile and South Africa (Parsons and Cuthbertson 1992; Peterson and Prasad 1998; Bellingham and others 2004). Specifically, we empirically tested four interlinked hypotheses:
-
1.
Broom invasion will influence decomposition rates of co-occurring species, with stronger impacts as density increases.
-
2.
The net direction of changes in decomposition rates will depend on the strength of different drivers and the extent to which they are affected by broom invasion. Specifically, we predict that changes in soil chemical properties (for example higher N-availability) will decrease decomposition rates, but that a shift to a bacterially dominated soil microbial community and an increase in litter quality (that is lower C/N ratios) of co-occurring species will increase decomposition rates.
-
3.
Co-occurring exotic species will show greater increases in litter quality, that is lower litter C/N ratios, and decomposition rates with increasing broom density than co-occurring native species;
-
4.
These changes will persist over the long-term (six years) despite broom removal.
Methods
Study Site and Field Experiment
The study site was located in St. James Conservation Area in the South Island of New Zealand (Lat. Long. = −42.460273, 172.830938; elevation = 800–900 m.a.s.l.; mean annual temperature = 10.3°C; mean annual rainfall = 1158 mm, Hanmer forest met station). Broom is one of the most widespread and abundant woody weed species in New Zealand and continues to expand its range and abundance. We worked along a diffuse broom invasion front in the St. James Conservation Area in which all stages of broom invasion occur, ranging from completely uninvaded to discrete near mono-dominant stands over relatively small spatial scales (for example 10–100 m). Vegetation at our study site is comprised of a mixture of native shrubs (for example Dracophyllum uniflorum, Leptospermum scoparium, Discaria toumatou), native grasses (for example Chionochloa flavescens subsp. brevis, Poa colensoi, Chionochloa macra, Rytidosperma setifolium) and exotic grasses (for example Agrostis capillaris, Anthoxanthum odoratum, Dactylis glomerata, Festuca rubra) and trees (for example Pseudotsuga menziesii, Pinus contorta). For this study, we focused on the responses of two exotic perennial C3 tufted grasses, Agrostis capillaris L. and Anthoxanthum odoratum L., and two native perennial C3 tussock grasses, Poa colensoi Hook.f. and Chionochloa flavescens subspecies Brevis Connor. These species were selected because they were the most common native and exotic grasses at the site. The two exotic grasses are native to Europe and invasive in many of the same regions as broom, for example North America (Shaben and Myers 2009).
The field experiment consisted of 20 permanently marked 20 m × 20 m plots, spaced at least 50 m apart and included the full range of broom abundance encountered at the site (aboveground broom mass ranged from 0 to 6.65 kg m−2). To locate plots randomly but to include the full gradient of broom abundance, we ran transects crossing areas of high and low density of broom, placing plot locations every 50 m and making preliminary density estimates of broom cover. These transects also included uninvaded areas of intact native vegetation. Four of the eight highest density broom plots received a broom removal treatment that was initiated in November 2008 and repeated in November 2011 by cutting aboveground broom stems to within 1 cm of the ground level and removing this material from the plot. Cut broom stems were immediately painted with the herbicide triclopyr 600 EC (200 ml l−1) to kill individual broom plants. Broom was left intact on the remaining plots. Broom density was measured on all plots in 2008 (prior to the removal treatment) and in 2014 (prior to the sampling and analyses in this study) by first determining total broom volume as the area of broom covered (m2) * average height of broom (m) in each plot and then converting this volume to biomass and density as:
-
1.
Total broom biomass (kg) = exp(−0.79 + 1.31 * log(total broom volume)) (Carswell and others 2001)
-
2.
Broom density (kg m−2) = Total broom biomass (kg)/area of plot (400 m2)
To test the ecosystem legacy of broom invasion, we compared four low broom density plots (mean broom density in 2014 = 0.04 ± 0.007 kg m−2) and four high broom density plots (mean broom density in 2014 = 4.8 ± 0.13 kg m−2) with the high broom density plots that received the removal treatment (mean broom density prior to removal in 2008 = 5.73 ± 0.42 kg m−2).
Experimental Design
Four controlled laboratory bioassay experiments were used to determine how litter decomposition rates were related to broom density and removal and to investigate the relative importance of differences in litter quality and soil properties. A litter quality experiment was designed to determine how changes in litter quality independently affected decomposition rates of litter and whether effects varied with co-occurring species identity. To do this, litter from each of four grass species (A. capillaris, A. odoratum, C. flavescens subsp. Brevis and P. colensoi) was collected from each plot across the broom density gradient and decomposed on a standardised soil (n = 80). The soil consisted of a homogenised mix of an equal volume of soil from five plots with medium broom density (2.2 ± 0.2 kg m−2). This soil was chosen as it was most likely to represent the range of edaphic conditions found across the site.
A soil property experiment aimed to determine how changes in soil properties independently influenced litter decomposition rates, and consisted of decomposing a standardised litter on soil sampled from each plot across the density gradient (n = 20). Dactylis glomerata L. litter was used as the standardised litter as it is a widespread invasive grass species in New Zealand that is present, but rare, at our study site, avoiding any confounding effects of home-field advantage (Ayres and others 2009). Senesced leaves of ten D. glomerata plants were collected from a grassland in Lincoln, New Zealand. Litter was air-dried and homogenised.
A litter + soil experiment was carried out to determine the net effects of broom-associated changes in litter quality and soil properties on decomposition rates. This experiment used litter from each of the four grass species (A. capillaris, A. odoratum, C. flavescens subsp. Brevis and P. colensoi) collected from each plot across the density gradient, decomposed on soil collected from the same plot (n = 80).
Finally, a reciprocal transplant experiment aimed to test how litter quality, soil properties and plant species identity interacted to affect decomposition rates across high broom density, low broom density and broom removal plots. It consisted of a fully factorial reciprocal design, where litter from each of the four grass species (A. capillaris, A. odoratum, C. flavescens subsp. Brevis and P. colensoi) was collected from each of the high broom density plots (n = 4), low broom density plots (n = 4) and broom removal plots (n = 4), and decomposed on soil collected from each of these plots (n = 144).
Soil and Litter Collection
Representative soil samples for the bioassays were taken from each plot by pooling five soil sub-samples (depth 10 cm, diameter 8 cm) collected from the centre of each plot and four orthogonal points 5 m from the centre. Fresh soil was sieved to 4 mm and stored at 4°C prior to analyses. KCl extractable N concentration (NO3 −-N and NH4 +-N) was determined colorimetrically in a segmented flow stream using an AutoAnalyser (Seal-Analytical). Net N-mineralisation rate was measured as the release of mineral N after incubation of soil samples (5 g) for 14 days at 25°C (Ross 1992). Soil moisture content, water-holding capacity (WHC) and pH (1:2.5, soil/water) were also determined for each soil sample. Litter of each of the four grass species (A. capillaris, A. odoratum, C. flavescens subsp. Brevis and P. colensoi) was collected from each plot. For each species, at least 10 g of senesced leaves was collected from at least five individual plants. Due to the timing of collection, the majority of senescent material available from A. odoratum was stem litter and not leaf litter, so stem litter was used for all analyses of this species. All litter was processed in the same way as the standardised litter described above (that is air-dried and homogenised). Litter C and N concentrations were measured on ground samples using an automated Dumas procedure on a Vario EL analyser (Elementar).
Microbial Community Analysis
We used phospholipid fatty acid (PLFA) analyses to characterise the soil microbial community, following methods described by Bardgett and others (1996) based on the methods of Bligh and Dyer (1959). We characterised the soil microbial community into broad microbial groups by summing the PLFAs (measured in μg g−1) indicative of each group. These included gram-positive bacteria (i-15:0, a-15:0, i-16:0, i-17:0 and a-17:0); gram-negative bacteria (cy-17:0, cy-19:0, 16:1w7c and 18:1w7c); actinomycetes (10Me16:0, 10Me17:0, 10Me18:0); fungi (18:2w6,9c); total bacteria (gram+, gram−, actinomycetes and the PLFA marker 15:0); and total PLFA (all markers described above plus the non-specific markers 14:0, 16:1w5c, 16:0, 18:1w9c, 18:0, 20:0, 20:4) as reported in the literature (Bardgett and others 1996; Frostegard and Baath 1996; Paul and Clark 1996; Zelles 1999; Waldrop and Firestone 2004). We calculated a gram-positive/gram-negative ratio and a relative measure of the fungal/bacterial ratio by dividing the main fungal PLFA marker (18:2w6,9c) by the sum of all bacterial PLFA markers.
Incubation Method
Decomposition rates were measured using standardised laboratory bioassays based on Wardle and others (2002). A 10-g dry weight equivalent soil sample, amended to a moisture content of 60% WHC, was placed in a 9-cm-diameter Petri dish. Nylon mesh (1 mm) was placed over the soil, and 0.3 g of air-dried leaf litter, cut into 10-mm fragments, was added. A sub-sample of leaf litter (0.5 g) was oven-dried at 60°C for 48 h and re-weighed. The equivalent oven-dry mass of the 0.3-g air-dried litter was used in all calculations. The Petri dishes were two-thirds sealed with tape and one-third sealed with Parafilm, this reduced moisture loss but allowed for gas exchange. Sealed Petri dishes were weighed and incubated at 22°C for 35 weeks, with soil moisture levels being regularly re-adjusted using de-ionised water. Upon harvest, all remaining litter was cleaned, dried at 60°C and weighed to determine dry mass remaining. Decomposition rate was determined as the percentage mass lost during incubation.
Data Analysis
All analyses were conducted in R (version 3.2.1). Relationships of broom density to soil properties, litter quality of each individual species and decomposition rates were analysed using regression. For each response variable, we tested linear, log-linear, quadratic and a Gaussian log-link generalised linear model, selecting the model with the lowest AIC (Akaike information criterion) value. For soil inorganic N concentrations, assumptions of normality and homoscedasticity were breached in all cases so a log10 (y) transformation was applied. A principal component analysis of proportional PLFA data was conducted using the rda function in R package VEGAN. The first PCA axis (PC1) explained 76% of the variation and a broken stick analysis using the PCAsignificance function in R package BiodiversityR showed this was the only significant axis. Best subsets multiple regression using the exhaustive search method of the regsubsets function from the Leaps R package and BIC as model selection criteria was used to determine which soil variables (N-mineralisation rate (log10 transformed due to non-normality), pH, WHC, fungal/bacterial ratio, Gram-positive/gram-negative bacterial ratio, and total PLFA) best explained the relationship between the decomposition rate of the standard substrate and the change in soil properties associated with increasing broom density (that is the soil properties experiment). Soil inorganic N concentrations and the PC1 axis of the PCA were left out of the multiple regression analysis due to co-linearity with other variables; however, when the PC1 axis was included in the model instead of the fungal/bacterial and gram +/gram− bacterial ratios, the same best subset of variables was produced (that is net N-mineralisation rate; Table S1).
One-way analysis of variance and Tukey HSD post hoc tests were used to analyse the differences in mean litter qualities, soil properties and decomposition rates between high broom density, low broom density and broom removal plots. A factorial ANOVA was used to assess the main and interaction effects of soil origin (that is soil collected from high broom density, low broom density or broom removal plots), litter origin (that is litter collected from high broom density, low broom density or broom removal plots) and species identity (that is species from which litter was collected) on decomposition rates in the reciprocal transplant experiment. Log10 (y) transformations were used if assumptions of normality or homoscedasticity were violated. Welch ANOVA tests and Games–Howell post hoc tests were used if ANOVAs still violated assumptions following transformations.
Results
Broom Density
Soil Chemical Properties, Soil Microbial Community and Litter Quality
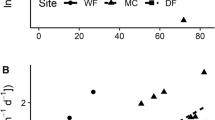
Soil inorganic N concentrations and net N-mineralisation rate both demonstrated strong nonlinear positive relationships with broom density (GLM, Gaussian log-link, (y + 1) ~ broom density, intercept = 0.08 ± 0.17 (standard error), broom density = 0.39 ± 0.03, t = 11.34, p < 0.001, Figure 1a; and log10(y + 0.1) = −0.61 + 0.51 * broom density; adj. R 2 = 0.75, p < 0.001, df = 14, Figure 1b, respectively). Soil inorganic N ranged from less than 0.03 mg N kg soil−1 (0.03 mg N kg soil−1 = detection limit) at low broom density to 129.4 mg N kg soil−1 at high broom density, whereas soil net N-mineralisation rate ranged from less than 0.03 to 10.2 mg N kg soil−1 day−1. Soil pH had a negative quadratic relationship with broom density (y = 4.95 + 0.06 * broom density − 0.04 * (broom density)2, adj. R 2 = 0.63, df = 13, p = 0.001; Figure 1c) and ranged from 5.26 at low broom density to 4.35 at high broom density.
Relationships between soil chemical properties and microbial community composition and density (kg m−2) of the invasive N-fixing shrub Cytisus scoparius (broom). Shaded regions represent 95% CIs. A Soil net N-mineralisation rate (N mg kg soil−1 day−1). B Log10 inorganic N concentrations (N mg kg soil−1). C Soil pH. D Fungal/bacterial ratio. E PLFA principal component axis 1 (PC1). F Gram-positive/gram-negative bacterial ratio.
The fungal/bacterial ratio and scores along the first principal component axis (PC1) of PLFA composition decreased linearly with broom density (y = 0.11–0.01 * broom density; adj. R 2 = 0.40, p < 0.01, df = 14, Figure 1d; and y = 0.075–0.039 * broom density; adj. R 2 = 0.57, p < 0.001, df = 14, Figure 1e, respectively), whereas the gram-positive/gram-negative bacterial ratio showed a significant nonlinear decrease (GLM, Gaussian log-link, y ~ broom density, intercept = −0.29 ± 0.03, broom density = −0.04 ± 0.01, t = −3.99, p = 0.001; Figure 1f). In contrast, total PLFA, total bacterial PLFA and total fungal PLFA did not vary significantly with broom density.
The litter C/N ratios of all species significantly decreased with broom density, although the form of the relationship varied. A. capillaris and C. flavescens both showed nonlinear quadratic relationships (y = 86.46–19.70 * broom density + 2.06 * (broom density)2, adj. R 2 = 0.67, df = 13, p < 0.001, Figure S1a; and y = 151.36–25.20 * broom density + 3.56 * (broom density)2, adj. R 2 = 0.28, df = 13, p < 0.05 Figure S1b, respectively), A. odoratum also demonstrated a nonlinear decrease (GLM, Gaussian log-link, y ~ broom density, intercept = 4.89 ± 0.08, broom density = −0.14 ± 0.04, t = −3.51, p = 0.004; Figure S1c), whereas P. colensoi showed a linear decrease (y = 81.50–7.76 * broom density, adj. R 2 = 0.55, p = 0.001, df = 14; Figure S1d). Overall, A. capillaris and P. colensoi showed lower mean C/N ratios (63.4 ± 5.8 and 66.6 ± 5.0, respectively) than A. odoratum (103.8 ± 9.1) and C. flavescens (129.2 ± 7.4) (F = 20.32, p < 0.0001). The C/N ratio of the standardised litter (D. glomerata) was 45.
Decomposition Rate
Despite significant decreases in litter C/N ratios for all species along the broom density gradient (Figure S1), decomposition rates of these litters did not change when decomposed on a standard soil (litter quality experiment; A. capillaris, p = 0.74; A. odoratum, p = 0.98; C. flavescens, p = 0.77; P. colensoi, p = 0.50). However, when a standard litter was decomposed on soil collected from across the broom density gradient (soil properties experiment), its decomposition rate showed a strong nonlinear negative relationship with broom density (GLM, Gaussian log-link, y ~ broom density, intercept = 4.35 ± 0.06, broom density = −0.08 ± 0.03, t = −3.30, p = 0.005; Figure 2a); the standard litter showed a mass loss of 92.9% on soil collected from low broom density plots but only 48.7% on soil from plots having high broom density. Net N-mineralisation rate was the best predictor of this decrease in decomposition rate (y = 76.80–29.26 * broom density, adj. R 2 = 0.43, p < 0.01, df = 14; Figure 2b and Table S1). In the litter + soil experiment, where each species’ litter collected from across the broom gradient was decomposed on soil collected from the same plot, decomposition rates were similar along the broom density gradient (A. capillaris, p = 0.94; A. odoratum, p = 0.82; C. flavescens, p = 0.32; P. colensoi, p = 0.64). On average, C. flavescens litter had significantly lower mean decomposition rates (36.3 ± 2.5) than litters of all other species: A. capillaris (44.2 ± 2.0), A. odoratum (44.6 ± 2.0) and P. colensoi (52.9 ± 2.5) (F = 11.13, p < 0.0001).
Broom Removal
Soil Chemical Properties, Soil Microbial Community and Litter Quality
Most soil, microbial and litter responses on broom removal plots were intermediate to values observed in the low broom and high broom density plots (Table 1). However, soil net N-mineralisation rates and inorganic N concentrations were significantly higher, and litter C/N ratios of A. capillaris and C. flavescens were significantly lower on broom removal plots compared to low broom density plots and showed no significant difference compared to high broom density plots (Table 1). A similar pattern was observed with soil pH, mean fungal/bacterial ratios and litter C/N ratios of A. odoratum, but these differences were only marginally significant (Table 1). Fungal, bacterial and total PLFAs did not respond to either increased broom density or broom removal. The gram+/gram− bacterial ratios and litter C/N ratios of P. colensoi were significantly lower on high broom density plots compared to low broom density plots (Table 1).
Litter Decomposition
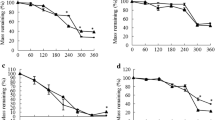
Despite shifts in mean litter C/N ratio for all species in relation to both broom invasion and removal (Table 1), there were no differences in mean decomposition rates of these litters on a standard soil (litter quality experiment; A. capillaris, F = 0.16, p = 0.86; A. odoratum, F = 0.48, p = 0.64; C. flavescens, F = 1.05, p = 0.40; P. colensoi, F = 0.86, p = 0.46). In contrast, the standardised litter had much lower decomposition rates on soils from either high broom density plots (53.3 ± 2.8%) or broom removal plots (53.4 ± 2.7%) than on soil from low broom density plots (84.3 ± 5.9%; F = 19.1, p < 0.001; Figure 3a; soil properties experiment). When litter collected from each species across low broom density, high broom density and broom removal plots was decomposed on soil from the same plot (litter + soil experiment), they showed no significant differences in decomposition rates (A. capillaris, F = 0.96, p = 0.42; A. odoratum, F = 0.15, p = 0.86; C. flavescens, F = 1.88, p = 0.21; P. colensoi, F = 0.20, p = 0.82).
Variation in decomposition (% mass loss) for A standardised litter response to soil origin (soil properties experiment), B litter across four co-occurring grass species response to soil origin (reciprocal transplant experiment) and C individual grass species in the reciprocal transplant experiment (Ac = A. capillaris, Ao = A. odoratum, Cf = C. flavescens, Pc = P. colensoi). Asterisks indicate significant difference from other means at p < 0.01 (Tukey HSD post hoc test). Box-and-whisker plots show individual data points, means, SEs and range of data.
Soil origin and species identity were the only significant factors that controlled decomposition rates in the reciprocal transplant experiment (Table S2); no significant interactions were found. Mean litter decomposition rates were on average significantly lower on soil from high broom density plots (35.4 ± 1.7) compared to soil from low broom density plots (49.0 ± 2.6) and broom removal plots (44.9 ± 1.9; F = 13.94, p < 0.0001; Figure 3b). The mean decomposition rate of P. colensoi litter (53.1 ± 2.7) was on average significantly higher than A. capillaris (40.0 ± 2.1) A. odoratum (42.3 ± 2.7) and C. flavescens (37.0 ± 2.3; F = 9.42, p < 0.0001; Figure 3c).
Discussion
Most studies focus on litter quality, particularly litter N-content, as the main driver of decomposition rates; here, we show that edaphic conditions may be more important in the context of N-fixer invasions. In our study, broom invasion substantially increased soil N-availability and this impact persisted, even 6 years post-broom removal. Of the soil properties measured, N-mineralisation rate appeared to be the best predictor of decomposition rates (Figure 2b), suggesting that the increasing soil N-availability associated with increasing broom density may be constraining litter decomposition. We also found that ecosystem impacts did not scale linearly with invasive plant density. Together these findings imply that heavily invaded areas may be experiencing larger and more permanent changes in ecosystem processes than currently expected, particularly in regard to reducing decomposition rates. Litter decomposition is a crucial process in biologically important nutrient cycles, and a reduction in decomposition rates is likely to alter the availability of key nutrients for plant growth, with implications for plant community composition, along with potentially increasing C sequestration in soil.
Broom Impacts on Decomposition Rates
Evidence that invader mediated changes to soil properties, particularly net N-mineralisation rate, were important in reducing decomposition rates comes from the soil properties experiment, where decomposition rates of the standard litter declined strongly as broom density increased (Figure 2a), and from the reciprocal transplant experiment, where soil from high broom density plots was associated with lower decomposition rates across all co-occurring species compared to soil from low broom density plots (Figure 3b). There are various mechanisms by which increasing soil N-availability can constrain decomposition rates, including direct suppression of ligninolytic enzyme activity (Gallo and others 2004; Ramirez and others 2012); the alleviation of microbial N requirements and consequent reduction in microbial N-mining of recalcitrant compounds, such as lignin (Craine and others 2007; Talbot and Treseder 2012); or a shift in microbial community composition as lignin decomposers become out-competed by microbes with high N-assimilation efficiency and cellulolytic capability (Couteaux and others 1995; Talbot and Treseder 2012). The shift in the soil microbial community, with an increase in bacteria over fungi (Figure 1d) and gram-negative over gram-positive bacteria (Figure 1f), is indicative of a change to faster cycling of N and raise the possibility that a guild of microbes, such as “N-miners” adapted to extremely low N-availability (<0.03 mg kg soil−1), may be out-competed and extirpated as soil N-availability increases due to broom invasion. It was, however, net N-mineralisation rate that best explained the decrease in decomposition rate, not indicators of the microbial community. This suggests that increasing N-availability may also be directly suppressing enzyme activity or altering microbial N requirements.
Litter C/N ratios were a poor predictor of decomposition rates in our study. This was unexpected as lower litter C/N ratios, such as those we observed in relation to broom invasion, are generally associated with higher decomposition rates (Perez-Harguindeguy and others 2000). Nevertheless, some N-addition experiments have also found no relationship between litter C/N ratios and decomposition rates (Knorr and others 2005; Knops and others 2007), whereas others have even reported reduced decomposition rates in relation to higher litter N concentrations (Van Diepen and others 2015). Increases in litter N concentrations can be accompanied by decreases in the concentrations of other important nutrients, including Ca, Mn and P, which may explain the reduced decomposition rates of these higher-N-content litters (Hobbie 2015; Van Diepen and others 2015). Such limitation of decomposition by other elements than N may also account for the lack of a relationship between litter C/N ratios and decomposition rates in our study. Furthermore, this could override the negative effect of increasing soil N-availability and therefore explain the lack of a relationship between litter decomposition and broom density in the litter + soil experiment.
Invader Density/Impact Relationships
Many of the relationships between invader density and various ecosystem properties were nonlinear, in line with other recent studies (Dickie and others 2011; Elgersma and Ehrenfeld 2011), strongly contradicting the current widespread assumption that invasive plant impacts are proportional to invader density (Parker and others 1999; Yokomizo and others 2009). This underlines the need to move away from end-point comparisons of invasive species abundance towards gradient analyses. Soil inorganic N concentrations, net N-mineralisation rate and pH all followed high-threshold density–impact curves (Yokomizo and others 2009) in response to increasing invader density (Figure 1a–c), whereby impact increases slowly at low invader density but rapidly at higher densities. As a consequence, at high invader densities, small changes in density result in large differences in impact. Should this type of density–impact relationship be common amongst other invasive N-fixer species, then it could explain some of the variation across studies that do not account for invader density. Conversely, the litter C/N ratios of A. capillaris, A. odoratum and C. flavescens all showed low-threshold density–impact curves (Figure S1a–c), whereby impact increases rapidly at low invader density levels. This may be because the additional inorganic N added to the soil at low broom density levels is rapidly acquired by co-occurring species. These findings imply that low levels of N-fixing invader density may have disproportionately large impacts on co-occurring species traits, in line with findings from other studies (Peltzer and others 2009; Elgersma and Ehrenfeld 2011). Native and exotic grass species responded in broadly similar ways to broom density, with the native grass P. colensoi showing a higher decomposition rate than the two exotic species in the reciprocal transplant experiment, contrary to our expectations. This suggests that species provenance may not necessarily be a useful indicator of the responses of co-occurring species to N-fixer invasions.
Broom Legacy Effects
Invader removal seems to have led to a slight recovery in ecosystem properties, as most soil properties and litter C/N ratios showed values intermediate to those measured on high and low broom density plots (Table 1). These intermediate values, however, were frequently not significantly different from those on high broom density plots. Litter decomposition rates partially recovered, with all co-occurring species showing very similar mean decomposition rates on broom removal soil and low broom density soil in the reciprocal transplant experiment (Figure 3b). The standard litter in the soil properties experiment, however, showed the opposite pattern, with equally reduced rates of decomposition on broom removal and high broom density soils compared to low broom density soils (Figure 3a). The discrepancy of results from these two experiments may be related to a combination of factors. Firstly, the standard litter had a C/N ratio of 45, whereas the other litters had mean C/N ratios of 63–129; secondly, the different plot types had variable microbial communities and N-availabilities (Table 1). The combination of these differences could have led to altered decomposer succession trajectories and ultimately divergent decomposition rates (Hobbie 2005). Other plant traits, such as litter lignin/N ratio, may also be driving litter decomposition rates, particularly of more recalcitrant litters (Taylor and others 1989). Additionally, the standard litter was rare across our plots in comparison with the other species litters, suggesting microbial adaptation to litter type may have modified results (Ayres and others 2009). The expectation would then, however, be that the litter from the more common species would decompose more rapidly than litter from the rarer species (Ayres and others 2009), which was not the case.
The lack of recovery in ecosystem N pools and fluxes that we observed six years after invasive N-fixer removal conforms to findings from previous studies (Von Holle and others 2013; Grove and others 2015). The longevity of such ecosystem N-enrichment is currently unknown, although higher N-availability has been detected 14 years after stands of another invasive N-fixer, Robinia pseudoacacia, were removed by a hurricane in the North-eastern US (Von Holle and others 2013). The soil microbial community also did not fully recover following broom removal (Table 1). These long-term legacies of broom invasion are likely to have important implications for other ecosystem properties, including potentially higher rates of primary productivity and reductions in biodiversity in response to higher N-availability.
Conclusion
The relationship between invader density and impact varied from linear to quadratic and exponential, depending on the impact being measured. This emphasises the need to quantify invader density to enable better comparison across studies and therefore facilitate invasive species management. The partial recovery of some ecosystem processes, including litter decomposition rates, following high-density broom removal, suggests this may be an effective management strategy. However, due to the long-term persistence of augmented ecosystem N pools and fluxes following broom removal we recommend prioritising broom removal before it attains high densities (>2 kg m−2). The decreases in decomposition rates and increases in ecosystem N pools and fluxes associated with N-fixer invasion in our study may increase soil carbon sequestration and primary productivity, while potentially decreasing biodiversity. If similar impacts occur in the many other ecosystems invaded by N-fixers worldwide then the implications for global nutrient cycling rates and biodiversity levels could be substantial. Nevertheless, our primary finding was that changes in soil properties associated with N-fixer invasion, particularly increasing N-availability, resulted in a soil environment that reduced decomposition rates of co-occurring species, whereas changes in litter quality had little effect. As far as we are aware, this is the first study to reveal the importance of this mechanism in driving invasive plant impacts on litter decomposition rates.
References
Aerts R. 1997. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–49.
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH. 2009. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biology and Biochemistry 41:606–10.
Bardgett RD, Hobbs PJ, Frostegard A. 1996. Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biology and Fertility of Soils 22:261–4.
Bardgett RD. 2005. The biology of soil: a community and ecosystem approach. Oxford: Oxford Univ. Press.
Bellingham PJ, Duncan RP, Lee WG, Buxton RP. 2004. Seedling growth rate and survival do not predict invasiveness in naturalized woody plants in New Zealand. Oikos 106:308–16.
Bligh, FOR EXAMPLE, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37.
Carswell F, Pearce G, Burrows L, Rogers G, Buxton RP, Dangeron F. 2001. Allometric relationships for estimating carbon content of shrublands. Task B2 in Contract report JNT0001/133 prepared for the Ministry for the Environment.
Castro-Díez P, Godoy O, Alonso A, Gallardo A, Saldaña A. 2014. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecology Letters 17:1–12.
Corbin JD, D’Antonio CM. 2012. Gone but not forgotten? Invasive plants’ legacies on community and ecosystem properties. Invasive Plant Science and Management 5:117–24.
Couteaux MM, Bottner P, Berg B. 1995. Litter decomposition, climate and litter quality. Trends in Ecology & Evolution 10:63–6.
Craine JM, Morrow C, Fierer N. 2007. Microbial nitrogen limitation increases decomposition. Ecology 88:2105–13.
Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecology letters 14:419–31.
de Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J. 2006. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biology and Biochemistry 38:2092–103.
Dickie IA, St John MG, Yeates GW, Morse CW, Bonner KI, Orwin K, Peltzer DA. 2014. Belowground legacies of Pinus contorta invasion and removal result in multiple mechanisms of invasional meltdown. AoB PLANTS 6:1–15.
Dickie IA, Yeates GW, St. John MG, Stevenson BA, Scott JT, Rillig MC, Peltzer DA, Orwin KH, Kirschbaum MUF, Hunt JE, Burrows LE, Barbour MM, Aislabie J. 2011. Ecosystem service and biodiversity trade-offs in two woody successions. Journal of Applied Ecology 48:926–34.
Ehrenfeld JG. 2003. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–23.
Ehrenfeld JG. 2010. Ecosystem consequences of biological invasions. Annual Review of Ecology, Evolution, and Systematics 41:59–80.
Elgersma KJ, Ehrenfeld JG. 2011. Linear and non-linear impacts of a non-native plant invasion on soil microbial community structure and function. Biological Invasions 13:757–68.
Frostegard A, Baath E. 1996. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils 22:59–65.
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR. 2004. Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microbial Ecology 48:218–29.
Grove S, Parker IM, Haubensak KA. 2015. Persistence of a soil legacy following removal of a nitrogen-fixing invader. Biological Invasions 17:2621–31.
Hobbie SE. 2000. Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–94.
Hobbie SE. 2005. Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–56.
Hobbie SE. 2015. Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends in Ecology & Evolution 30:357–63.
Hulme PE, Pyšek P, Jarošík V, Pergl J, Schaffner U, Vilà M. 2013. Bias and error in understanding plant invasion impacts. Trends in Ecology and Evolution 28:212–18.
Jackson MC, Ruiz-navarro A, Britton JR. 2014. Population density modifies the ecological impacts of invasive species. Oikos 124:880–7.
Knops JMH, Naeem S, Reich PB. 2007. The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Global Change Biology 13:1960–71.
Knorr M, Frey SDS, Curtis PS. 2005. Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–7.
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N. 2015. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proceedings of the National Academy of Sciences 112.
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S. 2003. Mechanisms underlying the impacts of exotic plant invasions. Proceedings of The Royal Society B Biological sciences 270:775–81.
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B. 2008. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytologist 177:706–14.
Lu M, Yang Y, Luo Y, Fang C, Zhou X, Chen J, Yang X, Li B. 2011. Responses of ecosystem nitrogen cycle to nitrogen addition: a meta-analysis. New Phytologist 189:1040–50.
Norton DA. 2009. Species invasions and the limits to restoration: learning from the New Zealand experience. Science 325:569–71.
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, von Holle B, Moyle PB, Byers JE, Goldwasser L. 1999. Impact: toward a framework for understanding the ecological effects of invader. Biological Invasions 1:3–19.
Parsons W, Cuthbertson E. 1992. Noxious weeds of Australia. Melbourne and Sydney: Inkata Press.
Paul EA, Clark FE. 1996. Soil microbiology and biochemistry. 2nd edn. San Diego: Academic Press.
Peltzer DA, Bellingham PJ, Kurokawa H, Walker LR, Wardle DA, Yeates GW. 2009. Punching above their weight: low-biomass non-native plant species alter soil properties during primary succession. Oikos 118:1001–14.
Peltzer DA, Kurokawa H, Wardle DA. 2016. Soil fertility and disturbance interact to drive contrasting responses of co-occurring native and non-native species. Ecology 97(15–0298):1.
Perez-Harguindeguy N, Diaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A. 2000. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil 218:21–30.
Peterson D, Prasad R. 1998. The biology of Canadian weeds. 109. Cytisus scoparius (L.) Link. Canadian Journal of Plant Science 78:497–504.
Ramirez KS, Craine JM, Fierer N. 2012. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biology 18:1918–27.
Ross DJ. 1992. Influence of sieve mesh size on estimates of microbial carbon and nitrogen by fumigation extraction procedures in soils under pasture. Soil Biology & Biochemistry 24:343–50.
Shaben J, Myers JH. 2009. Relationships between Scotch broom (Cytisus scoparius), soil nutrients, and plant diversity in the Garry oak savannah ecosystem. Plant Ecology 207:81–91.
St. John MG, Bellingham PJ, Walker LR, Orwin KH, Bonner KI, Dickie IA, Morse CW, Yeates GW, Wardle DA. 2012. Loss of a dominant nitrogen-fixing shrub in primary succession: consequences for plant and below-ground communities. Journal of Ecology 100:1074–84.
Talbot JM, Treseder KK. 2012. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 93:345–54.
Taylor B, Parkinson D, Parsons W. 1989. Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104.
Van Diepen LTA, Frey SD, Sthultz CM, Morrison EW, Minocha R, Pringle A. 2015. Changes in litter quality caused by simulated nitrogen deposition reinforce the N-induced suppression of litter decay. Ecosphere 6:1–16.
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14:702–8.
Von Holle B, Neill C, Largay EF, Budreski KA, Ozimec B, Clark SA, Lee K. 2013. Ecosystem legacy of the introduced N2-fixing tree Robinia pseudoacacia in a coastal forest. Oecologia 172:915–24.
Waldrop MP, Firestone MK. 2004. Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–84.
Wardle D, Bonner K, Barker G. 2002. Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Functional Ecology 16:585–95.
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH. 2004. Ecological linkages between aboveground and belowground biota. Science 304:1629–33.
Watt MS, Clinton PW, Whitehead D, Richardson B, Mason EG, Leckie AC. 2003. Above-ground biomass accumulation and nitrogen fixation of broom (Cytisus scoparius L.) growing with juvenile Pinus radiata on a dryland site. Forest Ecology and Management 184:93–104.
Wheeler CT, Perry DA, Helgerson O, Gordon JC. 1979. Winter fixation of nitrogen in Scotch broom (Cytisus scoparius). New Phytologist 82:697–701.
Williams PA. 1998. Response of broom (Cytisus scoparius) to control measures.
Xia J, Wan S. 2008. Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist 179:428–39.
Yokomizo H, Possingham HP, Thomas MB, Buckley YM. 2009. Managing the impact of invasive species: the value of knowing the density-impact curve. Ecological Applications 19:376–86.
Zelles L. 1999. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biology and Fertility of Soils 29:111–29.
Acknowledgements
Helen Quirk, Annette Ryan and Karen Boot are thanked for assistance in the laboratory. Larry Burrows, Chris Morse, Rowan Buxton, Kevin Drew, Brian Karl, Renske Terhurne, Jenny Hurst and Mark St. John are thanked for their efforts establishing, maintaining and measuring the field plots. We thank the New Zealand Department of Conservation for permission to work at the field site. AB is funded by a Lancaster University Faculty of Science and Technology studentship. This research was supported by the New Zealand Ministry of Business, Innovation and Employment Core funding to Crown Research Institutes and the New Zealand Ministry for the Environment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contributions
AB conceived of and designed study, performed research, analysed data and wrote the paper; KO conceived of and designed study, performed research and wrote the paper; DP performed research and wrote the paper; ID performed research and wrote the paper; NM performed research and wrote the paper; NO wrote the paper; CS wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Broadbent, A.A.D., Orwin, K.H., Peltzer, D.A. et al. Invasive N-fixer Impacts on Litter Decomposition Driven by Changes to Soil Properties Not Litter Quality. Ecosystems 20, 1151–1163 (2017). https://doi.org/10.1007/s10021-016-0099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0099-3