Abstract
Throughout most of the northern hemisphere, snow cover decreased in almost every winter month from 1967 to 2012. Because snow is an effective insulator, snow cover loss has likely enhanced soil freezing and the frequency of soil freeze–thaw cycles, which can disrupt soil nitrogen dynamics including the production of nitrous oxide (N2O). We used replicated automated gas flux chambers deployed in an annual cropping system in the upper Midwest US for three winters (December–March, 2011–2013) to examine the effects of snow removal and additions on N2O fluxes. Diminished snow cover resulted in increased N2O emissions each year; over the entire experiment, cumulative emissions in plots with snow removed were 69% higher than in ambient snow control plots and 95% higher than in plots that received additional snow (P < 0.001). Higher emissions coincided with a greater number of freeze–thaw cycles that broke up soil macroaggregates (250–8000 µm) and significantly increased soil inorganic nitrogen pools. We conclude that winters with less snow cover can be expected to accelerate N2O fluxes from agricultural soils subject to wintertime freezing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasing global surface temperatures, snow cover has decreased globally; in the northern hemisphere, snow cover has decreased in every winter month except November and December from 1967 to 2012 and will likely continue to decrease (IPCC 2013). Snow is an effective insulator, such that reduced snow cover can be expected to enhance soil freezing, increase the depth of frost, and perhaps increase the frequency of soil freeze–thaw cycles. Additionally, more extreme weather events may cause more frequent midwinter thaws in areas of agricultural importance such as the US Midwest (Isard and Schaetzl 1998; Pryor and others 2014).
Freeze–thaw cycles can strongly affect soil carbon (C) and nitrogen (N) dynamics, including emissions of nitrous oxide (N2O), a greenhouse gas with about 300 times the global warming potential of carbon dioxide (CO2) that also depletes stratospheric ozone. Agricultural soils account for approximately 60% of anthropogenic N2O emissions worldwide (IPCC 2007). In cold winter regions, high fluxes have been reported during spring thaws (for example, Goodroad and Keeney 1984; Christensen and Tiedje 1990; Wagner-Riddle and Thurtell 1998; Teepe and others 2001; Wolf and others 2010) and as well higher wintertime fluxes have been associated with soils more exposed to freeze–thaw events due to less snow cover (for example, Dorsch and others 2004; Groffman and others 2006; Maljanen and others 2007, 2009, 2010; Durán and others 2013).
Higher pulses of N2O following thaw have been attributed to (1) release of physically trapped N2O (Burton and Beauchamp 1994; Teepe and others 2001); (2) enhanced microbial activity upon release of dissolved organic C and N from aggregate disruption (Christensen and Christensen 1991; Sharma and others 2006) or upon disruption of microbial cells (DeLuca and others 1992; de Bruijn and others 2009) and fine roots (Groffman and others 2001; Tierney and others 2001); and especially (3) anaerobic conditions induced by thawing and consequent soil water saturation, conducive to denitrification (Furon and others 2008; de Bruijn and others 2009; Kim and others 2012; Risk and others 2014). In a recent review, Risk and others (2013) concluded that most N2O emitted on spring thaws is produced de novo rather than released from ice-trapped gas, underscoring the potential for midwinter freeze–thaw events to accelerate N2O production and release.
The importance of midwinter thaw events in situ is an important gap in our knowledge of N2O fluxes especially in agricultural soils (Venterea and others 2012), primarily because they are difficult to evaluate without high frequency measurements: thaw-induced emissions are typically highly pulsed, occurring within hours of a thaw, and in many climates and with increasing frequency, freeze–thaw events occur rapidly. In relatively few ecosystems do we have continuous sub-daily N2O flux measurements during winter; these include northern forests (for example, Loftfield and others 1992), cropland (for example, Wagner-Riddle and others 1996, 2007), and Mongolian steppe (Holst and others 2008; Wolf and others 2010), and in most of these studies, large pulses of N2O occur mainly at spring thaw. Snow cover presumably helps to moderate midwinter fluxes in such systems; it both protects microbes from sub-freezing temperatures that might otherwise halt N2O production (Sommerfeld and others 1993; Schürmann and others 2002) and as well protects soils from periodic thaws that would otherwise accelerate microbial activity (Christensen and Christensen 1991).
This moderating influence may be especially important in croplands. Unlike forest and grassland soils where wintertime N2O snow cover responses are tempered by vegetative cover (Groffman and others 2006; Maljanen and others 2007, 2009, 2010; Durán and others 2013), most annual cropland soils exposed to snow, unless fall-planted or cover-cropped, have little winter cover and thus N2O fluxes may be especially susceptible to snow cover changes. Very few studies have experimentally assessed the N2O response to reduced snow cover in annual crops (Dietzel and others 2011) and none at the sub-daily measurement frequency needed to overcome the uncertainty associated with weekly or longer sampling frequencies.
Here we report on a snow manipulation experiment designed to evaluate how future changes in snow cover may affect soil N2O fluxes in annual cropland soils, using an automated sampling system that captures fluxes four times per day. We hypothesize that (i) snow reduction will increase soil freeze–thaw cycles, which will (ii) increase N2O emissions throughout the winter possibly due to (iii) the breakup of soil aggregates and accelerated N mineralization. We hypothesize that snow addition will have opposite effects.
Materials and Methods
Site Description
During three winters (December–March 2010–2011, 2011–2012, 2012–2013, hereafter referred to as winters 2011, 2012, and 2013) we measured N2O emissions in an agricultural field in southwest Michigan, USA. The field was located at the Kellogg Biological Station (KBS) Long-Term Ecological Research (LTER) site (42°24′N, 85°24′W, elevation 288 m). Soils are Typic Hapludalfs, co-mingled Kalamazoo (fine-loamy, mixed, mesic) and Oshtemo (coarse-loamy, mixed, mesic) series loams developed on glacial outwash. Average Ap layer texture is 43% sand, 38% silt, and 19% clay, with 12.9 g C kg−1 and 1.31 g N kg−1 and a soil pH of 5.5. Annual precipitation (30-year mean) is 1027 mm with a snowfall of about 1.4 m and an average snow depth of 148 mm for days when snow is present. Mean annual temperature is 9.9°C ranging from a monthly mean of −4.2°C in January to 22.8°C in July (Robertson and Hamilton 2015). Figure S1 shows average snowfall, increasing winter temperatures and decreasing number of snow cover days over the past 63 years at KBS (see Supplementary material).
Experimental Design and Treatments
The experiment was a completely randomized design with three snow treatments: ambient snow cover, no-snow cover, and double-snow cover. In the no-snow treatment, after each snow event more than 95% of snow was carefully removed with a hand trowel without disturbing snow density; in the double-snow treatment, snow was carefully added to twice ambient levels so as to maintain existing snow density as closely as possible. Each treatment was replicated four times within a larger field for a total of twelve randomly located 4 × 4 m plots in which N2O fluxes were measured and soils sampled (described below). New plots were established each year within the field to avoid any residual effects of the prior year’s snow cover treatments.
The field containing treatment plots was managed as a no-till corn (Zea mays L.)–soybean (Glycine max L.)–winter wheat (Triticum aestivum L.) rotation according to regional norms (Robertson and Hamilton 2015). In 2011, the plots were in winter wheat (planted in November, 2010), in 2012, in corn (planted in May, 2012), and in 2013, in soybean (planted in May, 2013). All crops received conventional chemical inputs including pre- and post-emergence herbicide and fertilizers according to regional best management practices and integrated pest management protocols. Nitrogen fertilizer as urea ammonium nitrate was injected into the soil at ~10 cm depth at standard rates: wheat received 84 kg N ha−1 in early spring, corn received 168 kg N ha−1 split between planting in May and side-dressing in June, and soybeans received 7 kg N ha−1 at planting as starter N. Crop residues were left on the soil surface. There were no cover crops although fall-planted winter wheat had germinated and was present on all plots during winter 2011.
Nitrous Oxide (N2O) Emissions
Wintertime N2O fluxes (December–March) were measured in each plot with a fully automated flux chamber system based on that described in Breuer and others (2000) and Scheer and others (2013). Each of the twelve 16 m2 treatment plots contained a 50 cm × 50 cm × 38 cm high chamber mounted on a 15-cm-high base embedded 5 cm into the soil and left in place for the duration of each winter. When the treatment snow depth was higher than chamber height, 50-cm extensions were installed in all treatments and then removed following sublimation or snowmelt to maintain measurement sensitivity.
Each chamber was sampled four times per day at 6 h intervals. During sampling, the chamber lid was closed and headspace samples were pumped to a gas chromatograph located in a nearby trailer. N2O concentrations were measured four times from each chamber at intervals of approximately 30 min. N2O flux was calculated using linear regression of the N2O concentration (ppbv) against time for each of the four samples following temperature and pressure corrections. Three standards were injected at the beginning and end of each sampling period. The system also collected an air sample from each chamber prior to chamber closure. Gas samples were directly analyzed by gas chromatography (SRI 8610C with custom sample acquisition, Torrance, CA, USA). Gases were separated on a Restek packed HS-Q (3.7 m, 60/80 mesh) column in an oven at 60°C, and then N2O was analyzed with a 63Ni electron capture detector at 350°C with N2 5.0 UHP (Linde, USA) as the carrier gas.
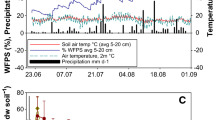
Soil temperature at 0–5 cm depth was measured every 30 min using HOBO pendant temperature data loggers (Onset Computer Corporation, Pocasset, MA, USA) installed in pairs in each plot. Loggers were calibrated against thermocouples (Omega Engineering, Inc., Stamford, CT, USA) in the lab, and differences were statistically indistinguishable over a range of −0.8 to 11°C (mean R 2 = 0.995, SD = 0.005, n = 8). Freezing-degree hours were to define the duration when soil temperature was below 0°C. One freeze–thaw cycle was defined as when soil temperature increase from below 0°C to above 0°C. Air temperature was recorded at a weather station within 100 m of the study site (http://lter.kbs.msu.edu/datatables/7). In addition, to approximate changes in the importance of wintertime vs. annual N2O emissions, we obtained growing season N2O emissions data from biweekly measurements of non-automated static chambers at four nearby plots with the same soil properties and identical agricultural management (http://lter.kbs.msu.edu/datatables/28).
Soil Inorganic Nitrogen
Total available N including ammonium (\( {\text{NH}}_{ 4}^{ + } \)) and nitrate (\( {\text{NO}}_{3}^{ - } \)) availability was estimated using in situ ion exchange resin strips to minimize sampling disturbance (Ruan and Robertson 2013). Three pairs of anion and cation resin strips (2.5 cm × 10 cm × 0.62 mm thick; GE Power & Water, Trevose, PA, USA) were buried directly to a soil depth of 12 cm in each treatment plot one day before the experiment commenced each winter and left in place for the season. After collection at the end of the season, 35 ml of 2.0 M KCl per resin strip were added to a polyethylene cup that was then shaken for 1 h at 40 rpm on an orbital shaker (IKA KS 501, Wilmington, NC, USA). A 5 ml extract was then analyzed for \( {\text{NH}}_{ 4}^{ + } \) and \( {\text{NO}}_{3}^{ - } \) on a continuous flow analyzer (Flow Solution IV, OI Analytical, College Station, TX, USA) using colorimetric techniques.
Water-Stable Aggregate Distribution
Soil aggregate distributions were determined before and after each winter season using the water-stable aggregate method (Elliott 1986; Grandy and Robertson 2006). On each sample date, five 12-cm-diameter soil cores (0–10 cm depth) were taken from each treatment plot, put through an 8-mm sieve and air dried at 25°C. Three 50-g air-dried subsamples from each plot were then wet-sieved in water through a series of 2000-, 250-, and 53-μm sieves to obtain four size fractions: 2000–8000 μm (large macroaggregates), 250–2000 μm (small macroaggregates), 53–250 μm (microaggregates), and less-than-53 μm (silt + clay particles). Before wet-sieving, soils were submerged in water on the surface of the 2000-μm sieve for 5 min. Then soils were sieved under water into a stainless steel pan by moving up and down over 2 min with a stroke length of 3 cm for 50 strokes. Soils remaining on the sieve were oven-dried at 60°C for 48 h. Soils passing the 2000 μm sieve and remaining in the pan were then wet-sieved through the 250-μm sieve (50 strokes) and then the 53-μm sieve (30 strokes). Sand content was determined by placing soil from each of the size classes larger than 53 μm in sodium hexametaphosphate (0.5%) and shaking for 48 h on a rotary shaker at 190 rpm and then sieving through a 53-μm sieve. The mean weight diameter (MWD) of sand-free aggregates was then calculated as the sum of products of the mean diameter of each size fraction and the proportion of the total dry sample weight (van Bavel 1949).
Data Analysis
We took one week before the first snow (usually early December) as a starting point and one week after the last snow (usually late March) as the ending point for each winter’s experimental period. Cumulative N2O fluxes over the period were calculated by linear interpolation of hourly fluxes between the every 6 h sample events. Statistical analysis was conducted in SAS 9.2 (SAS Institute, Cary, NC, USA). Treatment means (N2O fluxes, inorganic N, aggregate size, temperature, freezing hours and freeze–thaw cycles) were compared using one-way ANOVA with LSD in Proc Mixed at the α = 0.05 level. Linear regression between cumulative N2O fluxes and soil total available N was conducted in PROC REG. Normality of the residuals and homogeneity of variance assumptions were checked using stem-and-leaf box and normal probability plots of the residuals, and using Levene’s test. All data reported here are openly available on Dryad (Ruan and Robertson 2016).
Results
Snow Depth and Soil Temperature
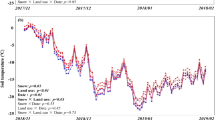
Snow fall for the winters of 2011–2013 totaled 942, 767, and 959 mm, respectively. These rates are lower than the average 1376-mm snowfall for the past 60 years (Figure S1A). Likewise, the total number of days with snow cover for the three winters were 58, 33, and 43 days, lower than the 60-year average of 66 days per winter (Figure 1B). Over the three winters, average air temperature ranged from −0.20 to −3.57°C, part of a general wintertime warming trend (Figure S1C).
Average soil temperatures (0–5 cm depth) in the no-snow treatment were 0.36 and 0.44°C colder than in the ambient and double-snow treatments, respectively, for all three winters (P < 0.05; Table 1 means). The no-snow treatment also experienced 29 and 47% more freezing-degree hours (P < 0.05) than the ambient and double-snow treatments and 2 and 2.3 times more freeze–thaw cycles (P < 0.05; Table 1 means). During periods with snow cover, soil temperatures under the double-snow treatment appeared to fluctuate less than in the other treatments, while soil temperatures in the no-snow treatment warmed more quickly in response to increased air temperature (Figs. 1, S2).
Soil N2O Fluxes
Soil N2O fluxes ranged from undetectable to 132 ± 21 μg N2O-N m−2h−1 during the three winters. N2O fluxes in the no-snow treatment fluctuated more widely than did those in the ambient and double-snow treatments (Figure 1, S2). High fluxes occurred mostly with the onset of warm periods when soil temperatures increased to above 0°C. For instance, soil temperature stayed below 0°C on December 30, 2010 and increased to above 4.8°C across all snow treatments on January 1, 2011. During these two days, N2O fluxes reached their seasonal peaks across all treatments (Figure 1). High fluxes tended to persist for a few hours to 1–2 days.
For all three winters, N2O emissions were significantly higher in the no-snow treatment than in the ambient and double-snow treatments, whereas there were no significant differences in N2O emissions between ambient and double-snow treatments (Figure 2A). On average, over all three winters, N2O emissions in the no-snow treatment (9.19 ± 0.61 μg N2O-N m−2 h−1) were 69 and 95% higher than in the ambient (5.43 ± 0.31 μg N2O-N m−2 h−1) and double-snow (4.71 ± 0.17 μg N2O-N m−2 h−1) treatments (P < 0.001).
Soil N2O emissions. In different snow treatments for the winters 2011–2013, A average wintertime N2O fluxes and B proportion of annual N2O emissions represented by wintertime fluxes. Error bars are standard errors (n = 4 replicate plots). Treatments within a season marked with different letters are significantly different from one another (P < 0.05). C Relationship between cumulative N2O fluxes and soil inorganic nitrogen availability measured with resin strips (0–10 cm depth) for all snow treatments over the winters 2011–2013 (R 2 = 0.37, P < 0.001, n = 36).
Snow removal significantly increased (P < 0.05) the apparent seasonal importance of wintertime N2O emissions regardless of annual crop type (Figure 2B). Assuming that the growing season flux is adequately captured by static chamber sampling, for the 2011 wheat year, wintertime fluxes in the no-snow treatment were 17.6 ± 1.5% of total annual fluxes, as compared to 12.1 ± 1.4% for the ambient and 9.0 ± 0.9% for the double-snow treatments. During the 2012 maize year, wintertime fluxes were 8.2 ± 1.4% in the no-snow treatment as compared to 5.1 ± 0.1% for the ambient and 4.3 ± 0.2% for the double-snow treatments. For the 2013 soybean year, the wintertime proportions of total annual flux was 18.9 ± 1.7% for the no-snow treatment, 14.2 ± 0.6% for the ambient treatment, and 13.2 ± 1.4% for the double-snow treatment. Overall, snow removal appeared to increase the wintertime proportion of annual N2O fluxes by 46% compared to ambient and by 77% compared to double snow. The difference between the ambient and double-snow treatment was not significant at the P < 0.05 level.
Soil Aggregation
Before each winter experiment commenced, there were no significant differences in any of the four aggregate size fractions among snow treatments (Figure S3). At the onset of the experiment in all three winters, the 2000–8000 μm macroaggregate plus 250–2000 μm macroaggregate fractions were on average about 0.7 g g−1 soil and the 53–250 μm microaggregate plus less-than-53 μm silt + clay fractions were about 0.3 g g−1. At winter’s end, soil macroaggregates in the no-snow treatment had declined significantly (P < 0.05) by 38% to 0.44 g g−1 on average as compared to pre-winter soils, whereas the microaggregate and silt + clay fraction increased by 98% to 0.56 g g−1 (Figure 3). In contrast, soil aggregate size did not significantly change in the ambient and double-snow treatments, although the macroaggregates fraction declined 11% and microaggregate and silt + clay fraction increased 28% in the ambient treatment. In addition, the mean weight diameter (MWD) of sand-free aggregates was significantly (P < 0.05) lower in the no-snow treatment than in the ambient and double-snow treatments for all three winters (Figure S4).
Soil aggregate dynamics. Proportional distribution of surface soil (0–10 cm depth) aggregates among size fractions in all snow treatments over winters 2011–2013. Macroaggregates include the 2000–8000 μm and 250–2000 μm size fractions; microaggregates include the 53–250 and <53 μm fractions. Values indicate average aggregate densities at the end of each winter; values in parentheses indicate the change from pre-winter densities (Figure S3). Asterisks next to parentheses indicate significant differences between pre- and post-winter densities (P < 0.05).
Soil Inorganic Nitrogen
Snow removal significantly increased both soil \( {\text{NH}}_{ 4}^{ + } \) and \( {\text{NO}}_{3}^{ - } \) availability over the winter (P < 0.05). Specifically, resin strip \( {\text{NO}}_{3}^{ - } \) concentrations in the no-snow treatment (70.3 ± 3.7 μg cm−2) were 22 and 45% higher than \( {\text{NO}}_{3}^{ - } \) concentrations in the ambient (57.8 ± 2.8 μg cm−2) and double-snow (48.5 ± 5.1 μg cm−2) treatments. Resin strip \( {\text{NH}}_{ 4}^{ + } \) concentrations were very low (<7.8 μg cm−2) compared to NO3 − concentrations, but even so \( {\text{NH}}_{ 4}^{ + } \) concentrations in the no-snow treatment were also significantly higher than \( {\text{NH}}_{ 4}^{ + } \) concentrations in the other treatments (P < 0.05).
Soil inorganic N concentrations explained 37% of mean cumulative N2O fluxes. N2O fluxes showed a positive linear relationship with the sum of \( {\text{NH}}_{ 4}^{ + } \) and \( {\text{NO}}_{3}^{ - } \) resin concentrations: N2O fluxes (g N ha−1) = (1.40 × available N (μg cm−2) + 61.5) (R 2 = 0.37, P < 0.001) (Figure 2C).
Discussion
Our results support the hypothesis that reduced snow cover can increase N2O emissions as a result of highly intermittent soil warming that increases the frequency of soil freeze–thaw cycles. On average, across all three winters, snow removal significantly stimulated N2O emissions by 69% relative to ambient conditions and by 95% relative to double-snow conditions. Fluxes were highly episodic, lasting for a period of hours to days following intermittent soil freeze–thaw cycles, which occurred 1.7 to 4 times more frequently in the no-snow treatments than in the ambient and double-snow treatments.
Snow removal also appears to have enhanced the importance of wintertime fluxes at our site. If static chambers reasonably estimate growing season N2O emissions (see below), then under ambient (non-snow removal) conditions winter fluxes made up about 9% of annual fluxes: about 12% of annual fluxes for the wheat in 2011, around 5% for the maize in 2012, and around 10% for the soybean in 2013. Snow removal increased these proportions almost 50%, on average.
Our ambient proportions are lower and in contrast to those estimated by Teepe and others (2000) for winter canola (Brassica napa) in Germany fall-fertilized at 200 kg N ha−1 y−1 and Johnson and others (2010) in Minnesota USA for alfalfa (Medicago sativa), where wintertime N2O emissions appeared to account for up to 58 and 65% of total annual emissions, respectively. Our lower estimate may be the result of a more frequent sampling interval (four measurements per day versus weekly for the canola and biweekly for the alfalfa studies) that better captures both low and high flux periods and avoids interpolation bias (Barton and others 2015; see Chamber Methodology below). Alternatively, N fixation (alfalfa) and fertilizer (canola) in these other studies may have stimulated more wintertime N2O production via added soil nitrogen. Another possibility is that our growing season fluxes are overestimated by the static chamber technique, in which case our wintertime proportions would be higher, though it seems more likely that our growing season fluxes may be underestimated because they are not consistently event based (Gelfand and others 2016).
Elevated N2O emissions in the no-snow treatment can likely be attributed to three main factors. First, increased freezing time enhances the mortality rate for microbes and fine roots, resulting in the release of labile organic carbon and N into the soil (DeLuca and others 1992; Groffman and others 2001; Tierney and others 2001). Snow removal decreased soil temperatures and increased freezing time in all three winters: the no-snow treatment had, on average, 283 more hours below 0°C than did the ambient treatment (Table 1). Likewise, the double-snow treatment had 132 fewer hours below 0°C. Loss of snow cover insulation resulted in freezing period differences that likely caused substrate availability differences among snow treatments. Heterotrophic denitrification, a dominant source of N2O in these soils (Ostrom and others 2010) is strongly affected by carbon availability (Robertson and Groffman 2015), especially during winter when thawed soils are saturated and largely anaerobic.
Second, the physical disruption of soil aggregates due to more freeze–thaw cycles where snow is absent may release previously protected organic matter to microbial attack (Christensen and Christensen 1991; van Bochove and others 2000), resulting in greater substrate availability where snow is absent. Soils in our no-snow treatment experienced twice the number of freeze–thaw cycles as ambient snow treatments, and this substantially reduced the density of macroaggregates—by 38% in the no-snow treatment, accompanied by a 98% increase in the microaggregate and silt + clay fraction. The breakup of large aggregates can also expose previously protected organic matter to oxygen concentrations more favorable to decomposition (Six and others 1999).
Freeze–thaw destruction of macroaggregates in situ has also been shown by others. In the Ah horizons of French alpine soils, Cécillon and others (2010) found that macroaggregates were diminished by 25% in plots with freeze–thaw events as compared to warmer frost-free plots. Edwards (2013) reported a 28% average decrease in larger aggregates (4750–9500 μm) together with a 33% increase in smaller aggregates (<500 μm) in arable soils of the Atlantic coast of Canada following multiple freeze–thaw cycles in the lab. On the other hand, Steinwig and others (2008) found no effects on aggregate size distributions in a snow removal treatment in forested soils at Hubbard Brook, NH, USA. They hypothesize that high water and organic matter contents together with slow rates of freezing can minimize structural disruption by freeze–thaw cycles in their forest soils.
A third factor contributing to elevated N2O emissions with snow removal is a greater availability of soil inorganic N: both NH4 + and NO3 − availability were higher in the no-snow treatment than in the ambient and double-snow treatments, likely the result of greater mineralization and nitrification rates due to increased freezing times and macroaggregate breakup as noted above. Nitrate, as an end product of nitrification and an electron acceptor for denitrification, is the best single predictor of N2O fluxes in these soils (Gelfand and Robertson 2015), such that increased N2O production might be expected with greater inorganic N availability. Moreover, Clark and others (2009) reported net N mineralization and nitrification in agricultural soils at sub-zero temperatures and inhibited N immobilization, which can also lead to more available N in frozen soils.
Inorganic N availability was assessed here with resin exchange strips, which measure both the static soil N pool and the N ions that flux through the mineral pool (Bowatte and others 2008). Resin strips can thus more readily represent temporally variable N availability than can conventional soil N extractions, and this may explain the difference between our N results and those of Groffman and others (2006), who did not find a snow removal effect on inorganic N availability.
Year-to-year differences in freeze–thaw cycles likely also contribute to normal variability in wintertime nitrate availability in these soils. The number of cycles in the ambient treatment varied from 12 to 28 during the three years of this study, with fewer cycles in 2012, the only year when the mean wintertime soil temperature was above 0 (Table 1). Also contributing to year-to-year nitrate variability will be management factors such as the prior crop with its specific fertilizer and residue inputs, although these differences cannot explain snow cover effects since all snow cover treatments occurred in the same cropping system each year.
Chamber Methodology
Our results provide a strong argument for using automated chambers with relatively high sampling frequency (multiple times per day vs. weekly to monthly manual chamber sampling) to investigate episodic N2O fluxes such as those that occur during midwinter soil thaws. Automated chambers have several advantages over manual chamber methods, especially in winter. First, they reduce soil disturbance that can be introduced by frequent manual sampling (Scheer and others 2013). Soil compaction can reduce porosity and increase water-filled pore space (WFPS), which in turn limits oxygen diffusion rates and results in an anaerobic state favorable for denitrification (van Groenigen and others 2005; Ball and others 2008).
Second, automated chambers can more precisely estimate total N2O emissions with a sub-daily sampling frequency that captures episodic events (Barton and others 2015). For example, in this study, a thaw-associated N2O peak of 118 ± 34 μg N2O-N m−2 h−1 was observed on December 31, 2010, in the ambient snow treatment. Two weeks later (on January 14, 2011), which is a commonly reported interval for N2O sampling (for example, Groffman and others 2006; Johnson and others 2010), the measured flux was 4.60 ± 4.31 μg N2O-N m−2 h−1. Linear interpolation between these sampling dates provides a cumulative flux estimate of 207 ± 53 g N2O-N ha−2 for the period, compared to 62.0 ± 7.9 g N2O-N ha−2 estimated by our sub-daily measurements. Thus using the two-week interval would have inappropriately increased the wintertime N2O contribution to the annual budget from 12.1 ± 1.4 to 22.4 ± 2.2%.
On the other hand, underestimation could as easily have been the case had other days been sampled, since most fluxes remained high for only 2–48 h. Parkin (2008), working in an chisel-plowed maize/soybean field in Iowa, found that the deviation of cumulative N2O flux increased as the sampling interval increased, and that sampling the data every 21 d yielded estimates ranging from +60 to −40% of the actual cumulative N2O flux.
Overall Significance
Overall, our finding that reduced snow in cropland soil accelerates N2O fluxes is in broad agreement with snow removal findings from northern hardwood and boreal upland forests, where weekly to monthly sampling has shown that snow removal can increase N2O fluxes by approximately 100% (Groffman and others 2006; Maljanen and others 2010). Similar trends occur in urban turfgrass (Durán and others 2013) and boreal hay fields (Maljanen and others 2007, 2009). Our study shows that north temperate annual croplands, with soils of relatively low organic matter and greater wintertime exposure to the effects of freeze–thaw cycles, are also affected by reduced snow cover and a consequently increased frequency of freeze–thaw cycles.
The particular significance of our results may rest with the emissions importance of agricultural soils in the global N2O cycle. Fertilized agricultural soils are the largest single source of anthropogenic N2O flux globally (IPCC 2007); the remainder comes from livestock waste management (from both confined and pastured animals), industrial activities, and biomass burning (IPCC 2014; Robertson 2014). Thus, any increase in the winter flux of N2O from northern agricultural soils can represent a significantly enlarged N2O source that is additionally subject to positive reinforcement as the climate warms.
Are higher wintertime N2O fluxes already occurring? Average snow cover at our site was 55% higher for the 60-year period preceding this study than it was during this study’s duration, and the total number of days with snow cover was 32% higher (Figure S2). For this site, then, higher wintertime N2O emissions are likely already occurring.
The global significance of past and future changes will depend on whether any increases in wintertime N2O from northern regions might be offset by reduced fluxes from more southerly regions, which would be expected to experience fewer freeze–thaw cycles. In large part, this will depend on the extent to which N2O production remains dependent on substrate made available by freeze–thaw cycles in these regions or whether other climate-related factors such as stronger wet-dry cycles or more active decomposers exert equivalent influence. The answers to these questions await further study.
Can increased wintertime fluxes from northern agricultural soils be avoided? More conservative N management that reduces the availability of surplus reactive N in soil is an important general strategy for combating accelerated N2O fluxes (Millar and others 2010). Another, specific to wintertime fluxes, is encouraging winter cover crops (Wagner-Riddle and Thurtell 1998) and maintaining crop residues that can trap and retain snow (Qiu and others 2011) and thereby abate the loss of snow cover that would otherwise occur. Cover crops would have the additional benefits of scavenging residual inorganic N (Syswerda and others 2012) and favoring soil aggregate stability (Liu and others 2005). That strategies to reduce surplus soil N can reduce N2O emissions during other parts of the year and, as well, reduce the loss of reactive N via other pathways (Robertson and Vitousek 2009) provides additional reasons to encourage such solutions now.
References
Ball BC, Crichton I, Horgan GW. 2008. Dynamics of upward and downward N2O and CO2 fluxes in ploughed or no-tilled soils in relation to water-filled pore space, compaction and crop presence. Soil Tillage Res 101:20–30.
Barton L, Wolf B, Rowlings D, Scheer C, Kiese R, Grace P, Stefanova K, Butterbach-Bahl K. 2015. Sampling frequency affects estimates of annual nitrous oxide fluxes. Sci Rep 5:15912.
Bowatte S, Tillman R, Carran A, Gillingham A, Scotter D. 2008. In situ ion exchange resin membrane (IEM) technique to measure soil mineral nitrogen dynamics in grazed pastures. Biol Fertil Soils 44:805–13.
Breuer L, Papen H, Butterbach-Bahl K. 2000. N2O emission from tropical forest soils of Australia. J Geophys Res 105:26353–67.
Burton DL, Beauchamp EG. 1994. Profile nitrous oxide and carbon dioxide concentrations in a soil subject to freezing. Soil Sci Soc Am J 58:115–22.
Cécillon L, de Mello NA, De Danieli S, Brun J-J. 2010. Soil macroaggregate dynamics in a mountain spatial climate gradient. Biogeochemistry 97:31–43.
Christensen S, Christensen BT. 1991. Organic matter available for denitrification in different soil fractions: effect of freeze/thaw cycles and straw disposal. J Soil Sci 42:637–47.
Christensen S, Tiedje JM. 1990. Brief and vigorous N2O production by soil at spring thaw. J Soil Sci 41:1–4.
Clark K, Chantigny MH, Angers DA, Rochette P, Parent L-É. 2009. Nitrogen transformations in cold and frozen agricultural soils following organic amendments. Soil Biol Biochem 41:348–56.
de Bruijn A, Butterbach-Bahl K, Blagodatsky S, Grote R. 2009. Model evaluation of different mechanisms driving freeze–thaw N2O emissions. Agric Ecosyst Environ 133:196–207.
DeLuca T, Keeney D, McCarty G. 1992. Effect of freeze-thaw events on mineralization of soil nitrogen. Biol Fertil Soils 14:116–20.
Dietzel R, Wolfe D, Thies JE. 2011. The influence of winter soil cover on spring nitrous oxide emissions from an agricultural soil. Soil Biol Biochem 43:1989–91.
Dorsch P, Palojarvi A, Mommertz S. 2004. Overwinter greenhouse gas fluxes in two contrasting agricultural habitats. Nutr Cycl Agroecosyst 70:117–33.
Durán J, Rodríguez A, Morse JL, Groffman PM. 2013. Winter climate change effects on soil C and N cycles in urban grasslands. Glob Change Biol 19:2826–37.
Edwards LM. 2013. The effects of soil freeze–thaw on soil aggregate breakdown and concomitant sediment flow in Prince Edward Island: a review. Can J Soil Sci 93:459–72.
Elliott ET. 1986. Aggregate structure and carbon nitrogen and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–33.
Furon AC, Wagner-Riddle C, Smith CR, Warland JS. 2008. Wavelet analysis of wintertime and spring thaw CO2 and N2O fluxes from agricultural fields. Agric For Meteorol 148:1305–17.
Gelfand I, Robertson GP. 2015. Mitigation of greenhouse gas emissions in agricultural ecosystems. In: Hamilton SK, Doll JE, Robertson GP, Eds. The ecology of agricultural landscapes: long-term research on the path to sustainability. New York: Oxford University Press. p 310–39.
Gelfand I, Shcherbak I, Millar N, Kravchenko AN, Robertson GP. 2016. Long-term nitrous oxide fluxes in annual and perennial agricultural and unmanaged ecosystems in the upper Midwest USA. Glob Change Biol. doi:10.1111/gcb.13426.
Goodroad LL, Keeney DR. 1984. Nitrous oxide emissions from soils during thawing. Can J Soil Sci 64:187–94.
Grandy AS, Robertson GP. 2006. Aggregation and organic matter protection following tillage of a previously uncultivated soil. Soil Sci Soc Am J 70:1398–406.
Groffman PM, Driscoll C, Fahey T, Hardy J, Fitzhugh R, Tierney G. 2001. Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56:135–50.
Groffman PM, Hardy JP, Driscoll CT, Fahey TJ. 2006. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Glob Change Biol 12:1748–60.
Holst J, Liu C, Yao Z, Brüggemann N, Zheng X, Giese M, Butterbach-Bahl K. 2008. Fluxes of nitrous oxide, methane and carbon dioxide during freezing–thawing cycles in an Inner Mongolian steppe. Plant Soil 308:105–17.
IPCC. 2007. Climate Change 2007: mitigation. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA, Eds. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge University Press.
IPCC. 2013. Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, Eds. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge University Press.
IPCC. 2014. Climate change 2014: mitigation of climate change. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow C, Zwickel T, Minx JC, Eds. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press: Cambridge.
Isard SA, Schaetzl RJ. 1998. Effects of winter weather conditions on soil freezing in southern Michigan. Phys Geogr 19:71–94.
Johnson JMF, Archer D, Barbour N. 2010. Greenhouse gas emission from contrasting management scenarios in the northern Corn Belt. Soil Sci Soc Am J 74:396–406.
Kim D-G, Vargas R, Bond-Lamberty B, Turetsky MR. 2012. Effects of soil rewetting and thawing on soil gas fluxes: a review of current literature and suggestions for future research. Biogeosciences 9:2459–83.
Liu A, Ma BL, Bomke AA. 2005. Effects of cover crops on soil aggregate stability, total organic carbon, and polysaccharides. Soil Sci Soc Am J 69:2041–8.
Loftfield NS, Brumme R, Beese F. 1992. Automated monitoring of nitrous-oxide and carbon-dioxide flux from forest soils. Soil Sci Soc Am J 56:1147–50.
Maljanen M, Alm J, Martikainen PJ, Repo T. 2010. Prolongation of soil frost resulting from reduced snow cover increases nitrous oxide emissions from boreal forest soil. Boreal Environ Res 15:34–42.
Maljanen M, Kohonen A-R, Virkajärvi P, Martikainen PJ. 2007. Fluxes and production of N2O, CO2, and CH4 in boreal agricultural soil during winter as affected by snow cover. Tellus 59B:853–9.
Maljanen M, Virkajärvi P, Hytönen J, Öquist M, Sparrman T, Martikainen PJ. 2009. Nitrous oxide production in boreal soils with variable organic matter content at low temperature—snow manipulation experiment. Biogeosciences 6:2461–73.
Millar N, Robertson GP, Grace PR, Gehl RJ, Hoben JP. 2010. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: an emissions reduction protocol for US Midwest agriculture. Mitig Adapt Strat Glob Change 15:185–204.
Ostrom NE, Sutka R, Ostrom PH, Grandy AS, Huizinga KH, Gandhi H, von Fisher JC, Robertson GP. 2010. Isotopologue data reveal bacterial denitrification as the primary source of N2O during a high flux event following cultivation of a native temperate grassland. Soil Biol Biochem 42:499–506.
Parkin TB. 2008. Effect of sampling frequency on estimates of cumulative nitrous oxide emissions. J Environ Qual 37:1390–5.
Pryor SC, Scavia D, Downer C, Gaden M, Iverson L, Nordstrom R, Patz J, Robertson GP. 2014. Chapter 18: Midwest. Melillo JM, Richmond TC, Yohe GW, editors. Climate Change Impacts in the United States: The Third National Climate Assessment: U.S. Global Change Research Program, p418–440.
Qiu H, Huggins DR, Wu JQ, Barber ME, McCool DK, Dun S. 2011. Residue management impacts on field-snow distribution and soil water storage. Transactions of the ASABE 54:1639–47.
Risk N, Snider D, Wagner-Riddle C. 2013. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can J Soil Sci 93:401–14.
Risk N, Wagner-Riddle C, Furon A, Warland J, Blodau C. 2014. Comparison of simultaneous soil profile N2O concentration and surface N2O flux measurements overwinter and at spring thaw in an agricultural soil. Soil Sci Soc Am J 78:180–93.
Robertson GP. 2014. Soil greenhouse gas emissions and their mitigation. In: Van Alfen N, Ed. Encyclopedia of agriculture and food systems. San Diego: Elsevier. p 185–96.
Robertson GP, Groffman PM. 2015. Nitrogen transformations. In: Paul EA, Ed. Soil microbiology, ecology, and biochemistry. Burlington: Academic Press. p 421–46.
Robertson GP, Hamilton SK. 2015. Long-term ecological research in agricultural landscapes at the Kellogg Biological Station LTER site: conceptual and experimental framework. In: Hamilton SK, Doll JE, Robertson GP, Eds. The ecology of agricultural landscapes: long-term research on the path to sustainability. New York: Oxford University Press. p 1–32.
Robertson GP, Vitousek PM. 2009. Nitrogen in agriculture: balancing the cost of an essential resource. Annu Rev Environ Resour 34:97–125.
Ruan L, Robertson GP. 2013. Initial nitrous oxide, carbon dioxide, and methane costs of converting Conservation Reserve Program grassland to row crops under no-till vs. conventional tillage. Glob Change Biol 19:2478–89.
Ruan L, Robertson GP. 2016. Data from: Reduced snow cover increases wintertime nitrous oxide (N2O) emissions from an agricultural soil in the upper U.S. Midwest. Ecosystems. http://dx.doi.org/10.5061/dryad.9c7s3.
Scheer C, Grace PR, Rowlings DW, Payero J. 2013. Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutr Cycl Agroecosyst 95:43–56.
Schürmann A, Mohn J, Bachofen R. 2002. N2O emissions from snow-covered soils in the Swiss Alps. Tellus B 54:134–42.
Sharma S, Szele Z, Schilling R, Munch JC, Schloter M. 2006. Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl Environ Microbiol 72:2148–54.
Six J, Elliott ET, Paustian K. 1999. Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J 63:1350–8.
Sommerfeld RA, Mosier AR, Musselman RC. 1993. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 361:140–2.
Steinweg JM, Fisk MC, McAlexander B, Groffman PM, Hardy JP. 2008. Experimental snowpack reduction alters organic matter and net N mineralization potential of soil macroaggregates in a northern hardwood forest. Biol Fertil Soils 45:1–10.
Syswerda SP, Basso B, Hamilton SK, Tausig JB, Robertson GP. 2012. Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA. Agric Ecosyst Environ 149:10–19.
Teepe R, Brumme R, Beese F. 2000. Nitrous oxide emissions from frozen soils under agricultural, fallow and forest land. Soil Biol Biochem 32:1807–10.
Teepe R, Brumme R, Beese F. 2001. Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biol Biochem 33:1269–75.
Tierney G, Fahey T, Groffman P, Hardy J, Fitzhugh R, Driscoll C. 2001. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 56:175–90.
van Bavel C. 1949. Mean weight diameter of soil aggregates as a statistical index of aggregation. Soil Sci Soc Am J 14:20–3.
van Bochove E, Prévost D, Pelletier F. 2000. Effects of freeze–thaw and soil structure on nitrous oxide produced in a clay soil. Soil Sci Soc Am J 64:1638–43.
van Groenigen JW, Kuikman PJ, de Groot WJM, Velthof GL. 2005. Nitrous oxide emission from urine-treated soil as influenced by urine composition and soil physical conditions. Soil Biol Biochem 37:463–73.
Venterea RT, Halvorson AD, Kitchen N, Liebig MA, Cavigelli MA, Grosso SJD, Motavalli PP, Nelson KA, Spokas KA, Singh BP, Stewart CE, Ranaivoson A, Strock J, Collins H. 2012. Challenges and opportunities for mitigating nitrous oxide emissions from fertilized cropping systems. Front Ecol Environ 10:562–70.
Wagner-Riddle C, Furon A, McLaughlin NL, Lee I, Barbeau J, Jayasundara S, Parkin G, Von Bertoldi P, Warland J. 2007. Intensive measurement of nitrous oxide emissions from a corn-soybean-wheat rotation under two contrasting management systems over 5 years. Glob Change Biol 13:1722–36.
Wagner-Riddle C, Thurtell GW. 1998. Nitrous oxide emissions from agricultural fields during winter and spring thaw as affected by management practices. Nutr Cycl Agroecosyst 52:151–63.
Wagner-Riddle C, Thurtell GW, Ling KM, Kidd GE, Beauchamp EG. 1996. Nitrous oxide and carbon dioxide fluxes from a bare soil using a micrometeorological approach. J Environ Qual 25:989–97.
Wolf B, Zheng X, Brüggemann N, Chen W, Dannenmann M, Han X, Sutton MA, Wu H, Yao Z, Butterbach-Bahl K. 2010. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 464:881–4.
Acknowledgements
We thank K. Kahmark and S. Bohm for help with sensors and the automated chamber system, C. McMinn, J. Simmons, and S. Vander Wulp for additional assistance in the field and lab, and J. Schuette for the help with graphics. S.K Hamilton and A.N. Kravchenko made many helpful suggestions and comments on an earlier draft. Financial support was provided by the US Department of Energy’s Office of Science (DE-FCO2-07ER64494) and Office of Energy Efficiency and Renewable Energy (DE-ACO5-76RL01830), the US National Science Foundation LTER Program (DEB 1027253) and MSU AgBioResearch.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
LR and GPR both conceived and designed the study; LR performed the research and most analyses; both LR and GPR wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ruan, L., Robertson, G.P. Reduced Snow Cover Increases Wintertime Nitrous Oxide (N2O) Emissions from an Agricultural Soil in the Upper U.S. Midwest. Ecosystems 20, 917–927 (2017). https://doi.org/10.1007/s10021-016-0077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0077-9