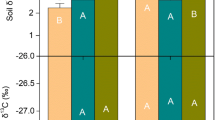
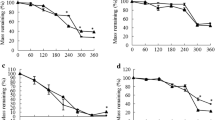
Abstract
Symbiotic N2-fixing tree species can accelerate ecosystem N dynamics through decomposition feedbacks via both direct and indirect pathways. Direct pathways include the production of readily decomposed leaf litter and increased N supply to decomposers, whereas indirect pathways include increased tissue N and altered detrital dynamics of non-fixing vegetation. To evaluate the relative importance of direct and indirect pathways, we compared 3-year decomposition and N dynamics of N2-fixing red alder leaf litter (2.34% N) to both low-N (0.68% N) and high-N (1.21% N) litter of non-fixing Douglas-fir, and decomposed each litter source in four forests dominated by either red alder or Douglas-fir. We also used experimental N fertilization of decomposition plots to assess elevated N availability as a potential mechanism of N2-fixer effects on litter mass loss and N dynamics. Direct effects of N2-fixing red alder on decomposition occurred primarily as faster N release from red alder than Douglas-fir litter. Direct increases in N supply to decomposers via experimental N fertilization did not stimulate decomposition of either species litter. Fixed N indirectly influenced detrital dynamics by increasing Douglas-fir tissue and litter N concentrations, which accelerated litter N release without accelerating mass loss. By increasing soil N, tissue N, and the rate of N release from litter of non-fixers, we conclude that N2-fixing vegetation can indirectly foster plant–soil feedbacks that contribute to the persistence of elevated N availability in terrestrial ecosystems.



Similar content being viewed by others
References
Adair EC, Hobbie SE, Hobbie RK. 2010. Single-pool exponential decomposition models: potential pitfalls in their use in ecological studies. Ecology 91:1225–36.
Austin AT, Ballare CL. 2010. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci USA 107:4618–22.
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH. 2009. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–10.
Berg B, Matzner E. 1997. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25.
Binkley D. 2003. Seven decades of stand development in mixed and pure stands of conifers and nitrogen-fixing red alder. Can J For Res 33:2274–9.
Binkley D, Sollins P, Bell R, Sachs D, Myrold D. 1992. Biogeochemistry of adjacent conifer and alder-conifer stands. Ecology 73:2022–33.
Binkley D, Cromack K Jr, Baker DD. 1994. Nitrogen fixation by red alder: biology, rates, and controls. In: Hibbs DE, DeBell DS, Tarrant RF, Eds. The biology and management of red alder. Corvallis: Oregon State University Press. p 57–72.
Brown KR, Courtin PJ. 2003. Effects of phosphorus fertilization and liming on growth, mineral nutrition, and gas exchange of Alnus rubra seedlings grown in soils from mature alluvial Alnus stands. Can J For Res 33:2089–96.
Chapin FS, Walker LR, Fastie CL, Sharman LC. 1994. Mechanisms of primary succession following deglaciation at Glacier Bay Alaska. Ecol Monogr 64:149–75.
Chappell HN, Prescott CE, Vesterdal L. 1999. Long-term effects of nitrogen fertilization on nitrogen availability in coastal Douglas-fir forest floors. Soil Soc Am J 63:1448–54.
Cleveland CR, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, von Fischer JC, Elseroad AR, Wasson MF. 1999. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob Biogeochem C 13:623–45.
Cole DW, Compton JE, Edmonds RL, Homann PS, Van Miegroet H. 1995. Comparison of carbon accumulation in Douglas-fir and red alder forests. In: McFee WW, Kelly JM, Eds. Carbon forms and functions in forest soils. Madison, WI: Soil Science Society of America Inc. p 527–46.
Compton JE, Cole DW. 2001. Fate and effects of phosphorus additions in soils under N2-fixing red alder. Biogeochemistry 53(225–247):2001.
Compton JE, Church MR, Larned ST, Hogsett WE. 2003. Nitrogen export from forested watersheds in the Oregon Coast Range: role of N2-fixing red alder. Ecosystems 6:773–85.
Edmonds RL. 1980. Litter decomposition and nutrient release in Douglas-fir, red alder, western hemlock, and Pacific silver fir ecosystems in western Washington. Can J For Res 10:327–37.
Edmonds RL, Tuttle KM. 2010. Red alder leaf decomposition and nutrient release in alder and conifer riparian patches in western Washington, USA. For Ecol Manag 259:2375–81.
Forrester DI, Schortemeyer M, Stock WD, Bauhus J, Khanna PK, Cowie AL. 2007. Assessing nitrogen fixation in mixed- and single-species plantations of Eucalyptus globulus and Acacia mearnsii. Tree Physiology 27:1319–28.
Franklin JF, Dyrness CT. 1973. Natural vegetation of Oregon and Washington. Corvallis, OR: Oregon State University Press.
Fyles JW, Fyles IH. 1993. Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J For Res 23:358–61.
Goering HK, Van Soest PJ. 1970. Forage fiber analysis (apparatus, reagents, procedures, and some applications). Agricultural Research Service, United States Department of Agriculture. Agriculture Handbook No. 379.
Gosz JR. 1981. Nitrogen cycling in coniferous ecosystems. In: Clark FE, Roswall T, Eds. Terrestrial nitrogen cycles. Ecol Bull 33:405–426.
Harmon ME, Baker GA, Spycher G, Greene SE. 1990. Leaf-litter decomposition in the Picea/Tsuga forests of Olympic National Park, Washington, U.S.A. For Ecol Manag 31:55–66.
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS, LIDET. 2009. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Glob Change Biol 15:1320–38.
He X, Critchley C, Bledsoe C. 2003. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit Rev Plant Sci 22:531–67.
Hopmans P, Chappell HN. 1994. Growth response of young, thinned Douglas-fir stands to nitrogen fertilizer in relation to soil properties and tree nutrition. Can J For Res 24:1684–8.
Houlton BZ, Wang YP, Vitousek PM, Field CB. 2008. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–30.
Johnson DW. 1992. Nitrogen retention in forest soils. J Environ Qual 21:1–12.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Menge DN, Levin SA, Hedin LO. 2009. Facultative versus obligate nitrogen fixation strategies and their ecosystem consequences. Am Nat 174:465–77.
Moore TR, Trofymow JA, Prescott CE, Titus BD, CIDET Working Group. 2011. Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forests. Plant Soil 339:163–75.
Perakis SS, Matkins JJ, Hibbs DE. 2012. Interactions of tissue and fertilizer nitrogen on decomposition dynamics of lignin-rich conifer litter. Ecosphere 3(6):art54.
Perakis SS, Kellogg CH. 2007. Imprint of oaks on nitrogen availability and δ15 N in California grassland-savanna: a case of enhanced N inputs? Plant Ecol 191:209–20.
Perakis SS, Sinkhorn ER. 2011. Biogeochemistry of a temperate forest nitrogen gradient. Ecology 92:1481–91.
Perakis SS, Sinkhorn ER, Compton JE. 2011. δ15 N constraints on long-term nitrogen balances in temperate forests. Oecologia 167:793–807.
Prescott CE. 1994. Does nitrogen availability control rates of litter decomposition? Plant Soil 168–169:83–8.
Prescott CE. 2005. Do rates of litter decomposition tell us anything we really need to know? For Ecol Manage 220(1–3):66.
Prescott CE, Taylor BR, Parsons WFJ, Durall DM, Parkinson D. 1993a. Nutrient release from decomposing litter in Rocky Mountain coniferous forests: influence of nutrient availability. Can J For Res 23:1576–86.
Prescott CE, McDonald MA, Gessel SP, Kimmins JP. 1993b. Long-term effects of sewage sludge and inorganic fertilizer on nutrient turnover in litter in a coastal Douglas-fir forest. For Ecol Manage 59:149–64.
Prescott CE, Zabek LM, Staley CL, Kabzems R. 2000. Decomposition of broadleaf and needle litter in forest of British Columbia: influences of litter type, forest type, and litter mixtures. Can J For Res 30:1742–50.
Rastetter EB, Vitousek PM, Field C, Shaver GR, Herbert D, Ågren GI. 2001. Resource optimization and symbiotic N fixation. Ecosystems 4:369–88.
Richards AE, Forrester DI, Bauhus J, Scherer-Lorenzen M. 2010. The influence of mixed tree plantations on the nutrition of individual species: a review. Tree Physiol 30:1192–208.
SAS Institute inc. 2009. SAS 9.2 User’s Guide. Cary, NC: SAS Institute inc.
Scott EE, Perakis SS, Hibbs DE. 2008. δ15 N patterns of Douglas-fir and red alder riparian forests in the Oregon Coast Range. For Sci 54:140–7.
Valachovic YS, Caldwell BA, Cromack K Jr, Griffiths RP. 2004. Leaf litter chemistry controls on decomposition of Pacific Northwest trees and shrubs. Can J For Res 34:2131–47.
Van Kessel C, Farrell RE, Roskoski JP, Keane KM. 1994. Recycling of the naturally-occurring 15N in an established stand of Leucaena leucocephala. Soil Biol Biochem 26:757–62.
Vitousek PM. 2004. Nutrient cycling and limitation. Princeton, NJ: Princeton University Press.
Vitousek PM, Walker LR. 1989. Biological invasion by Myrica faya: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–65.
Wigington PJ, Church MR, Strickland TC, Eshleman KN, Van Sickle J. 1998. Autumn chemistry of Oregon Coast Range streams. J Am Water Resour Assoc 34:1035–49.
Zavitkovski J, Newton M. 1971. Litterfall and litter accumulation in red alder stands in western Oregon. Plant Soil 35:257–68.
Acknowledgments
The authors thank Chris Catricala, Aaron Thiel, and Manuela Huso for laboratory and statistical assistance, and Mark Harmon, Cindy Prescott, and two anonymous reviewers for helpful comments on the manuscript. This research was supported by the Oregon State University Forest Research Laboratory and NSF-DEB 0346837. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
SSP and DEH conceived of the study, JJM and SSP performed research, JJM and SSP analyzed data, and SSP, JJM, and DEH wrote the paper.
An erratum to this article is available at http://dx.doi.org/10.1007/s10021-014-9746-8.
Rights and permissions
About this article
Cite this article
Perakis, S.S., Matkins, J.J. & Hibbs, D.E. N2-Fixing Red Alder Indirectly Accelerates Ecosystem Nitrogen Cycling. Ecosystems 15, 1182–1193 (2012). https://doi.org/10.1007/s10021-012-9579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9579-2