Abstract
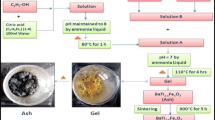
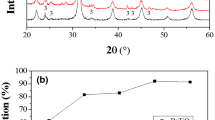
Bulk structure and surface texture of BaTiO3 materials synthesized at low temperature using sol-gel technique have been investigated. The materials were produced by the pyrolysis of an xerogel precursor of the tentative formula BaTiO3-x(CH3COO)2x, which was prepared using 1:1 molar ratio of barium acetate and titanium oxyacetate solution. The present method avoids using alkali-metal hydroxides (as a hydrolyzing agent), and thus produces an alkali-metal free precursor. The decomposition course of the xerogel at the onset of formation of crystalline BaTiO3 was probed applying thermogravimetry (TG), differential scanning calorimetery (DSC), Fourier-transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) techniques. Results indicated that most of the precursor weight loss occurs below 400°C, with the formation of titania rich intermediates. However, it was not until the temperature reached ≥600°C that well crystallized BaTiO3 was produced. The specific surface area and porosity were assessed for BaTiO3 produced at 600–1000°C using N2 adsorption at liquid N2 temperature.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 21 July 1998 / Reviewed and accepted: 30 September 1998
Rights and permissions
About this article
Cite this article
Khalil, K. Low temperature evolution of crystalline BaTiO3 from alkali-metal free precursor using sol-gel process. Mat Res Innovat 2, 256–262 (1999). https://doi.org/10.1007/s100190050095
Issue Date:
DOI: https://doi.org/10.1007/s100190050095