Abstract.
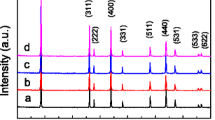
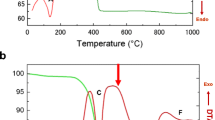
Electrochemical reductive dissolution of Li–Mn–O and Li–Fe–Mn–O spinels and Li+ extraction/insertion in these oxides were performed using voltammetry of microparticles. Both electrochemical reactions are sensitive to the Fe/(Fe+Mn) ratio, specific surface area, Li content in tetrahedral positions, and Mn valence, and can be used for electrochemical analysis of the homogeneity of the elemental and phase composition of synthetic samples. The peak potential (E P) of the reductive dissolution of the Li–Mn–O spinel is directly proportional to the logarithm of the specific surface area. E P of Li–Fe–Mn–O spinels is mainly controlled by the Fe/(Fe+Mn) ratio. Li+ insertion/extraction can be performed with Mn-rich Li–Fe–Mn–O spinels in aqueous solution under an ambient atmosphere and it is sensitive to the regularity of the spinel structure, in particularly to the amount of Li in tetrahedral positions and the Mn valence.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Grygar, T., Bezdička, P., Piszora, P. et al. Electrochemical reactivity of Li–Mn–O and Li–Fe–Mn–O spinels. J Solid State Electrochem 5, 487–494 (2001). https://doi.org/10.1007/s100080100193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s100080100193