Abstract
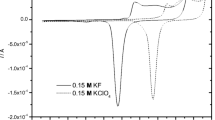
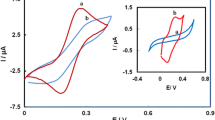
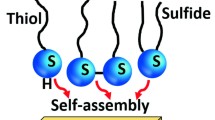
The formation and characterization of self-assembled monolayers of organosulfur compounds like alkanethiols and dialkyl (di)sulfides on metal surfaces such as gold are areas of current research interest. The presence of an aromatic ring in a thiol molecule can enhance the binding between Au and the thiol, resulting in the formation of compact and impervious self-assembled monolayers. The formation of a monolayer of 2-mercaptobenzothiazole (MBT), containing an aromatic group with a fused thiazole ring but no long alkyl chain, is achieved on a gold electrode surface. Voltammetric investigations of ferro/ferricyanide and ferrous/ferric redox systems carried out on this Au|MBT electrode are reported. Further, the possibility of using such an Au|MBT electrode to distinguish between inner and outer sphere electron transfer reactions is indicated.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 2 January 1998 / Accepted: 14 May 1998
Rights and permissions
About this article
Cite this article
Berchmans, S., Yegnaraman, V. & Prabhakara Rao, G. Self-assembled monolayers on electrode surfaces: a probe for redox kinetics. J Solid State Electrochem 3, 52–54 (1998). https://doi.org/10.1007/s100080050130
Issue Date:
DOI: https://doi.org/10.1007/s100080050130