Abstract
Fenton-based processes, chemical and electrochemical, have attracted the interest of industrial and academic researchers for wastewater treatment. However, the deficiency of rigorous comparison between different methods, including assessment of their impact on the environment, has hindered their large-scale application. This study reports for the first time on the sustainability of raw textile wastewater treatment through two sequential processes, Coagulation-Flocculation-Fenton-Neutralization (CF-F-N) and Coagulation-Flocculation-Electro-Fenton-Neutralization (CF-EF-N), based on Life Cycle Assessment (LCA) approach. The CF-F-N and CF-EF-N were optimized at laboratory scale and compared through LCA, using the IPCC-2013 and ReCiPe-2016 midpoint and endpoint methods. The highest CO2 emissions relied on the wastewater primary treatment by CF. This due to the high amount of hazardous sludge generated and the technology necessary for its disposal (i.e., 16.89 kg CO2-Eq/FU for underground deposit in security cells or 47.52 kg CO2-Eq/FU for incineration) as well as the consumption of reagents required for the treatment (alum, 7.72 kg CO2-Eq/FU; and slaked lime, 5.56 kg CO2-Eq/FU). Regarding the sequential processes, the EF-N presented lower carbon footprint (CFP) than the F-N (14.74 kg CO2-Eq/FU vs. 20.74 kg CO2-Eq/FU). Electricity (87.02% of the total CFP) and reagents (88.63% of the total CFP) denoted the main environmental hotspot during the EF-N and F-N, respectively. The EF-N, compared to the F-N, had an inferior incidence in 14 of the 18 impact categories analyzed using the ReCiPe-2016 method at the midpoint level. This is the result of low consumption of reagents and auxiliary chemicals. The electricity was also found as main environmental hotspot of the EF-N. The ReCiPe-2016 method at the endpoint level showed that the EF-N resulted in lower environmental load in all impact categories. The economic performance (11.91 USD/m3 for CF-EF-N vs. 13.66 USD/m3 for CF-F-N) and LCA demonstrated the competitiveness of the electrochemical sequential process compared to the chemical one. The CF-EF-N can be considered more environmentally sustainable technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainability is managing resources to meet the needs of the present without compromising the future [1]. This concept has guided the way in which new technologies, goods, and services should be developed, in order to guarantee adequate integration of technical, economic, and environmental aspects in decision-making [2]. In general, the first two aspects have defined the application of technologies for wastewater treatment. However, the addition of the environmental variable in the analysis of the global sustainability of the process can be decisive [3].
The textile and clothing industry plays an important role in the global economy. In 2023, the worldwide textile market was valued at USD 1837.27 billion; by 2030, it is expected to increase to a compound annual growth rate of 7.6% in profits [4]. In Colombia, it is one of the most traditional industrial sectors, with production centers in practically the entire national territory. During the last decade, the textile industry has represented close to 1% of the country’s gross domestic product (GDP) and between 8 and 9% of industrial GDP, incorporating more than 4000 companies related to the manufacture of textile products and more than 1.5 million of jobs [5]. However, it is not possible to hide the negative impact that its wastewater can generate on the environment. It has been estimated that the textile industry is responsible for 17–20% of the total pollution generated by the industrial sector in the world [6], mostly due to high consumption of water (200–400 m3/ton of processed fiber). In 2021, in Colombia, the wastewater generated by the manufacturing industry was around 189 million cubic meters, of which approximately 6% corresponded to the “Textiles, clothing, footwear and leather” sector. Of this percentage, 16% did not receive any type of treatment that would allow adequate conditions for the discharge of wastewater into water bodies [7].
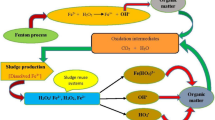
A wide variety of physical, chemical, and biological processes, as well as their sequential couplings, have been investigated for the treatment of textile effluents [6, 8,9,10,11,12,13]. Among them, advanced oxidation processes (AOPs) constitute an alternative for the treatment of toxic and recalcitrant pollutants [14,15,16,17,18,19] and have been successfully applied for the removal of a wide variety of organic dyes [10, 20,21,22,23,24]. AOPs comprise a series of methods that are based on the in situ formation of radical species (e.g., hydroxyl radicals (HO•, E° = 2.8 V), sulfate radicals (SO4•–, E° = 2.5–3.1 V)), which have a high oxidative power capable of degrading complex substances (including dyes) until their mineralization to CO2 and H2O [25,26,27]. Their generation involve the use of strong oxidizing agents (e.g., hydrogen peroxide (H2O2), ozone (O3), persulfate or peroxymonosulfate), catalysts (e.g., iron ions, electrodes, carbonaceous materials), and/or solar or ultraviolet radiation, and ultrasound. Among several, the Fenton process has been applied efficiently for the degradation of various substances, including dyes [24, 28]. This is due to its ease of operation and implementation in existing wastewater treatment plants, in autonomous or hybrid systems, and in operation under room temperature and pressure conditions, which facilitates the design and assembly of reactors and necessary infrastructure. Its high capacity to eliminate color and reduce organic load lies in the generation of hydroxyl radicals; short-lived, non-selective, and highly reactive oxidizing agents; decomposition products of H2O2 mediated by the ferrous ion (Fe2+). The application of the Fenton-Neutralization process for the treatment of textile effluent highly contaminated with Acid Black 194 (AB194) dye (ITWW), under optimal operating conditions [29], allowed the elimination of approximately 89% COD, 75% TOC, almost total discoloration, allowing compliance with the maximum permissible discharge limits into surface water bodies or public sewage systems [30] and reuse of the treated effluent [31]. However, the resulting effluent was still toxic. On the other hand, when implementing the Fenton process on a large scale, some difficulties may arise that must be considered: narrow operating range of pH (2.8–3.0) to avoid the precipitation of Fe2+ and the appearance of secondary reactions; significant quantities of chemicals necessary to acidify wastewater before treatment and neutralize effluents prior to discharge; production and accumulation of sludge that must be removed and disposed of at the end of the treatment; and safety problems related to the transportation, storage, and handling of H2O2 [32], which in turn implies a series of environmental impacts associated with the use of this reagent [33] and relatively high operating costs associated with the consumption of reagents. Thus, to reduce both operational costs and environmental impacts derived from the production, transportation, use, and storage of H2O2, the Electro-Fenton (EF) process emerged as an alternative to the traditional Fenton process [33,34,35,36]. This consists of the in situ electrogeneration of H2O2 from a cathodic reduction of the oxygen dissolved in wastewater treated under acidic conditions. Thus, H2O2 in combination with Fe2+ produces HO• radicals responsible for the oxidation of organic contaminants [34]. In principle, the EF process is characterized by a lower consumption of reagents, due to the continuous electrogeneration of H2O2 in the medium and the regeneration of Fe2+ on the cathode surface (therefore, a lower generation of sludge), and an increase in the reaction efficiency caused by the constant supply of HO• radicals, coming from the Fenton reaction and the electrolysis of water on the anodic surface [32].
Numerous studies have focused on the search for optimal operating conditions for AOPs to achieve complete mineralization and/or detoxification of colored effluents [37,38,39,40,41,42]. Unfortunately, only a few addressed industrial wastewater, focusing on the efficiency of contaminant removal, and not many reported on the operating costs [25, 43,44,45,46,47]. The possibility of combined operation between physico-chemical processes or biological systems with AOPs, in different configurations, has also been raised in the open literature as a strategy to overcome the limitations of each type of treatment [15, 48,49,50,51,52]. One of these configurations proposes the sequential operation (i.e., each process as a treatment stage), to be carried out in separate units, based on the coupling of the physico-chemical process (pretreatment) and an AOP as a definitive method. However, there is very little information, if any, on the possible effect of the implementation of these processes on the environment (impact of: energy consumption, production of construction materials and chemical reagents, gaseous emissions, transformation products, generation of solid waste, etc.). In this context, the Life Cycle Assessment (LCA) can be considered an appropriate tool to know in advance the potential environmental impacts that the treatment process can generate on the ecosystem [53,54,55]. They can be calculated considering all inventory elements (e.g., from cradle-to-gate) using universal databases [56, 57].
LCA was previously applied to analyze the environmental impact of different AOPs for the treatment of petrochemical [58], municipal [59], olive mills [60], and pharmaceutical and personal care products wastewater [61], among others. However, the analysis focused only on the evaluation of the environmental impacts generated by a single unit operation or by a limited set of units. Regarding colored effluents, the LCA of AOPs was implemented in autonomous (Fenton for Reactive Orange 16 (RO16) dye [62]) and hybrid (Electrocoagulation-Ozonation for Direct Blue 71 [63]) systems. However, these effluents were simulated, not raw industrial wastewater. To the best of our knowledge, only Nakhate et al. [64] reported on the life cycle assessment of a raw textile wastewater treatment plant composed of seven operational units, including an ozonation process.
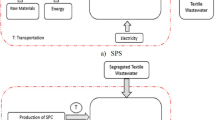
Thus, this work reports for the first time on the sustainability of raw textile wastewater treatment through two sequential Fenton-based processes, chemical and electrochemical, based on LCA approach. The wastewater, highly contaminated with AB194 dye among others, originated from the dyeing stage of a textile plant located in the Coffee Region of Colombia (South America). The sequential processes, Coagulation-Flocculation-Fenton-Neutralization (CF-F-N) and Coagulation-Flocculation-Electro-Fenton-Neutralization (CF-EF-N), operated under optimal working conditions, as reported previously [65, 66]. Thus, the objective and scope of this work is to assess the environmental footprint of the CF-F-N and CF-EF-N processes, to identify their advantages and disadvantages in terms of their environmental impacts, to compare them, and to provide feedback on the most sustainable process for future applications on a large-scale in textile wastewater treatment facilities. In short term, this will also allow to more closely evaluate the viability of electrochemical technology (EF) as alternative solution to conventional treatment (F) to ensure water availability and sustainable management and sanitation for all (within Sustainable Development Goal (SDG)-6). In the long term, the objective is to promote sustainable industrial development and encourage innovation (within SDG-9), seeking innovative solutions to mitigate the environmental impacts of the textile industry (within SDG-12) and to protect the environment from additional burdens. For this purpose, the LCA was carried out in large-scale virtual reactors. In order to assess the overall performance from both an environmental and technical point of view, the production and transportation of reagents, construction materials and electrodes, the production and consumption of electricity, as well as the voltage transformation (from high to medium voltage), the residual by-products and their final disposal, and the emissions resulting from the different stages of treatment were considered.
Methodology
Industrial textile wastewater
In this study, the industrial effluent came from the dyeing stage of a textile plant located in the Colombian Coffee Belt (South America). To obtain a representative sample of the industrial effluent (ITWW), three samplings were carried out, monthly, directly from the homogenization tank with a total capacity of 2.5 m3, as described in detail in our previous work [67]. In each sampling, a composite sample of 50 L was taken. This was obtained by mixing five specific samples of 10 L each, manually taken from the homogenization tank every 15 min. For the sampling, preservation, storage and management of ITWW, the guidelines and protocols of the Colombian Institute of Hydrology, Meteorology and Environmental Studies were followed [68,69,70,71].
Prior to the implementation of the sequential processes (F-N and EF-N), the ITWW was treated by Coagulation-Flocculation assisted by slaked lime (CF), as described previously [67]. The CF experiments were performed at laboratory scale according to the ASTM D2035-19 [72] in a jar testing equipment (MaquinLab Electronic®, MDJf6, Bogotá, Colombia) with six equal compartments with a capacity of 2 L each. After completing each jar test, the treated effluent was filtered (using a medium retention glass fiber filter, 11 µm, grade 1 (Munktell Ahlztrom®, 09471 Bärenstein, Germany), and the supernatant obtained in each jar was analyzed in triplicate [73] (Supplementary Material Table S1). Under optimal operating conditions (Alum: [Al2(SO4)3·14H2O] = 16.09 g/L, slaked lime [Ca(OH)2] = 5.16 g/L), CF removed approximately 62% of the COD, 55% of BOD5 and 97% of the color with a total cost of 5.99 USD/m3 (Supplementary Material Table S1). However, the values of the physicochemical parameters of the treated effluent did not allow compliance with the maximum permissible discharge limits into surface water bodies or public sewage systems [30]. Furthermore, the CF supernatant was still toxic.
Experimental tests
This work is based on results of experimental studies developed by members of our research group, as reported elsewhere [65,66,67]. From these, the optimal operating conditions of the CF, CF-F-N, and CF-EF-N were obtained. The main characteristics of these systems along with the specific operating conditions are given in Supplementary Material Table S2. The removal yields of organic compounds as well as operating costs were also included. All experimental results reported in this study are average values from three independent experiments.
The treatment of the supernatant from the CF through F-N and EF-N processes, under optimal operating conditions (Supplementary Material Table S2), allowed the elimination of approximately 66% of the COD, 60% of the TOC, and practically its total discoloration (greater efficiency of the EF process), thus letting compliance with the maximum permissible discharge limits required by current national environmental regulations [30]. Furthermore, the two effluents were found to be non-toxic.
Environmental impact assessment of treatment alternatives
The environmental impact assessment was carried out following ISO 14040 and 14,044 standards [53, 74]. Both the primary treatment using CF and the sequential processes, CF-F-N and CF-EF-N, implemented under optimized operating conditions (Supplementary Material Table S2), were analyzed considering the four main stages: (1) goal and scope, (2) inventory analysis, (3) life cycle impact assessment, and (4) interpretation of results [75].
Goal and scope definition
This study aims to evaluate and compare, through LCA, the environmental performance of two sequential processes, chemical (CF-F-N) and electrochemical (CF-EF-N), for the treatment of an ITWW highly contaminated with AB194. This took into account a cradle-to-gate approach. For comparison purposes, the functional unit (FU) selected for the LCA was 1 m3 of ITWW (to be treated through the primary CF process) and 1 m3 of ITWW-CF (to be treated through sequential processes, CF-F-N and CF-EF-N), under optimal operating conditions, thus guaranteeing compliance with current Colombian environmental legislation regarding the residual COD content (400 mg/L), among others [30]. The resulting effluents from both CF-F-N and CF-EF-N were non-toxic (from Artemia salina biotests).
For the design of large-scale reactors, the required quantities of chemicals, the specific optimal operating conditions, and the energy inputs necessary to carry out the treatment were considered, based on our previous research and other bibliographic reports. For coagulation-flocculation, a mixing tank is considered, preceded by a chemical feeding tank. The sludge generated during CF is settled at the end of the process and disposed of. The CF supernatant is fed to the sequential process. For the Fenton process, a circular concrete stirred tank (batch process) is considered, preceded by a chemical feeding tank. At the end of the Fenton process, the required amount of slaked lime is added to neutralize the content of the reactor. The sludge generated (F-N) is settled and disposed of. For the Electro-Fenton process, a circular concrete tank provided with a carbon felt sheet as cathode and a boron-doped diamond plate as anode is considered. To induce the electric field inside the reactor, a DC power source is connected to the electrodes, with a monopolar configuration arranged in parallel, under galvanostatic operation mode. Oxygen is supplied from the bottom of the electrochemical reactor through a diffused aeration system. At the end of the EF process, the content of the reactor is neutralized by adding caustic soda, and the sludge generated (EF-N) is settled and disposed of. All tanks include a motor for stirring the wastewater during the oxidation and neutralization processes.
For the LCA, the following main assumptions were made:
-
Location of the treatment plant: city of Manizales, Colombia, South America (latitude = 5° 4′ 12.99″ N, longitude = 75° 30′ 49.74″ W).
-
A useful life of 20 years and operation of the FU for 330 days a year (1 cycle per day).
-
The main activities (system boundaries of each process) within the LCA (as shown in Fig. 1):
-
1.
Production of all required reagents and materials for running the treatment (alum, slaked lime, H2O2, FeSO4, H2SO4 (for pH adjustment), NaOH or slaked lime (for neutralization), NaCl (for electrical conductivity adjustment), electrodes and oxygen supply).
-
2.
Production of construction materials for the development of the reactor infrastructure (concrete, cement, brick, sand, gravel, cast iron, high-density polyethylene (HDPE), polyester (e.g., PET)).
-
3.
Residuals (disposal of sludge generated (solid waste) and CO2 emissions into the atmosphere, a product of mineralization through sequential processes). The sludge generated in the primary process (CF) was considered hazardous waste. In this stage, more than 95% of the AB194 dye was removed from the textile wastewater, in addition to oil and grease, phenols, hydrocarbons, metals, and metalloids, among others. On the other hand, the sludge generated in sequential processes (F and EF) was considered an inert waste to be disposed of in a landfill. In these oxidative processes, the residual dye, among other contaminants, was oxidized; therefore, the sludge was composed mainly of iron and slaked lime. Finally, CO2 emissions into the atmosphere were defined and quantified based on the efficiency of the mineralization of the organic matter, in terms of TOC, through sequential oxidative processes.
-
4.
Transportation of all reagents, electrodes and construction materials to the treatment plant as well as sludge to its final disposal (within the metropolitan area). Most reagents and construction materials were considered to be produced near to the treatment plant (local providers). The materials and electrodes produced outside the country were transported by ship to the port of Buenaventura (latitude = 3° 52′ 48.36″ N; longitude = -77° 01′ 52.18″ W) and then were transported by road in EURO3 (16–32 metric ton) vehicles to Manizales city (Table 1).
-
5.
Electricity consumption (pumping, stirring, aeration, electrochemical cell supply) and voltage transformation from high to medium tension. In Colombia, electricity is supplied mainly by two sources, hydroelectric plants (70%) and thermal plants operated with coal (15%) and natural gas (15%) [76].
-
- Losses of materials and reagents of 10% and 8%, respectively, of the total amount required by the FU were assumed, as is usually observed in construction works.
Life cycle inventory (LCI)
The LCI information (Table 1) used in this work originated from the following sources: (1) laboratory-scale tests under optimal operating conditions; (2) scientific literature; and (3) professionals (know-how) of different fields of study. All data necessary for the life cycle of reagents, materials, transport, electricity, and sludge management were obtained from the Ecoinvent v 3.3 database [77] and evaluated using the OpenLCA 1.11 (GreenDelta©, Berlin, Germany) software.
Life cycle impact assessment (LCIA)
The LCIA was developed using OpenLCA software version 1.11 (GreenDelta©, Berlin, Germany). To evaluate the environmental impacts of sequential processes, the methods of the Intergovernmental Panel on Climate Change (IPCC-2013) and ReCiPe-2016 v1.1 were used. The IPCC-2013 method involves the performance of a process in terms of CO2 equivalent emissions (CO2-Eq) to the atmosphere over a time horizon of 100 years (Global Warming Potential, GWP-100) [75]. This impact category is considered more appropriate in making decisions related to the sustainability of different processes [3]. On the other hand, the ReCiPe-2016 method considers 18 environmental impact categories at the midpoint level, focused on specific environmental issues. The 18 categories considered in this LCA are shown in Table 2. At the endpoint level, these 18 categories could be grouped into 3 global impact categories: damage to human health (HH, unit: year, represents the years that are lost or that a person is disabled due to a disease or accident), damage to ecosystem quality (ED, unit: species per year, represents the local species loss integrated over the time), and damage to resource availability (RA, unit: US dollar, represents the extra costs involved for mineral and fossil resource extraction in the future) [78]. At the midpoint and endpoint levels, ReCiPe-2016 v1.1 takes into consideration three perspectives: individualistic, hierarchical, and egalitarian, to group different sources of uncertainty and options. The hierarchical perspective was used here, which is based on scientific consensus on the time frame and plausibility of impact mechanisms [78].
Life cycle interpretation
This last stage of the LCA study considered the LCI and LCIA results of the evaluated treatment processes to discuss their sustainability and the main environmental hotspots that need to be addressed (i.e., use of chemical reagents, energetic consumption) for future large-scale applications.
Results and discussion
The IPCC-2013 method
The LCA of the sequential processes, CF-F-N and CF-EF-N, was developed in two stages. CF was initially evaluated as primary treatment. Since CF is not an oxidative process, its sludge can be highly toxic as organic contaminants and other substances are transferred from the liquid to the solid phase. In the specific case of the ITWW, object of this study, the CF eliminated approximately 95% of the AB194 dye, among others (oils, fats, phenols, hydrocarbons, metals and metalloids). Therefore, the resulting sludge contains all these substances and in significant quantities, especially the dye (approximately 1000 ppm). AB194 is toxic to aquatic life (Medium Lethal Concentration, LC50, for fish of approx.10 mg/L) and presents long-lasting effects, according to the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) [79]. As stated by the Decree 1076 of 2015 [80], a waste is considered dangerous if it has corrosive, reactive, explosive, toxic, flammable, infectious, or radioactive characteristics that may cause risk or damage to human health and the environment. From this reason, its treatment and disposal must be carried out in accordance with its main characteristics. Thus, CF sludge was considered dangerous, and two alternatives for its management and disposal were analyzed: incineration (scenario 1, S1) and underground deposit in security cells (scenario 2, S2). The total carbon footprint (CFP) of CF-S1 was 47.52 kg CO2-Eq/FU, of which 70.3% (33.40 kg CO2-Eq/FU) corresponded to sludge incineration (Fig. 2). This is due to its high demand in terms of energy and stages for the elimination of polluting and toxic compounds. On the other hand, the CFP of CF-S2 was 16.89 kg CO2-Eq/FU, of which 16.39% (2.77 kg CO2-Eq/FU) corresponded to sludge disposal in security cells (underground deposit) (Fig. 2). After residuals, the consumption of reagents was identified as the stage that most contributes to the CFP (alum, 7.72 kg CO2-Eq/FU; slaked lime, 5.56 kg CO2-Eq/FU), which is explained by the high requirement of these supplies (16.09 kg alum/FU and 5.16 kg slaked lime/FU). Although alum is one of the most used coagulants for the treatment of textile wastewater, mostly due to its low cost and high performance, its use can result in an increase in the residual aluminum content in both the treated effluent and the generated sludge. Furthermore, in high concentrations, it can be toxic to aquatic organisms, contribute to the acidification of water bodies, and affect soil quality [81]. The other stages considered in the LCA (infrastructure, electricity and transportation) resulted in a contribution of 0.84 kg CO2-Eq/FU. The electricity consumption is not significant due to the short operating times and low stirring velocity required. Thus, two main environmental hotspots were identified: reagent consumption and residuals. Therefore, for future large-scale applications, the exploration of other alternatives, such as the use of different coagulants or flocculants and/or other technologies for primary treatment (e.g., electrocoagulation), may be a possibility to consider in decision-making.
The LCA applied to the sequential processes showed that the CFP of EF-N was significantly lower than that of F-N (14.74 kg CO2-Eq/FU vs. 20.74 kg CO2-Eq/FU) (Fig. 3). Note that in the case of EF-N, electricity presented the most significant contribution to the CFP (87.02%: 12.83 kg CO2-Eq /FU). This considering that in Colombia, the energy matrix is diversified and comes mainly from a renewable source (ca. 70% from hydroelectric plants) [76]. In fact, voltage transformation (from high to medium tension), rather than electricity consumption, turned out to be one of the critical stages in the life cycle of EF-N process (Fig. 3a), representing 67.15% (8.61 kg CO2-Eq/FU) of the CFP associated with electricity and 58.44% of the total CFP.
Thus, the voltage transformation is of special interest since this generates emissions of sulfur hexafluoride (SF6, extremely potent greenhouse gas, GWP-100 = 23,500 [82]), ozone (O3), and nitrogenous compounds (NOx). In addition, it involves loss of electricity and requires non-renewable materials for its operation (copper, steel, aluminum, porcelain, insulating materials, etc.) [83]. Although it is outside the scope of this study, to reduce its negative effect on CFP, it is necessary to decrease emissions of SF6 and other polluting compounds with high warming potential [82]. This can be achieved by improving and/or updating equipment and ensuring its proper functioning through preventive maintenance. Furthermore, the use of renewable energy sources that do not involve high voltage transformations, such as independent systems (photovoltaic), can also reduce the negative impact associated with energy consumption. This can potentially increase the competitiveness of electrochemical methods in comparison with the chemical ones [56]. In an alternative scenario, in which the electricity required by the EF-N process comes entirely from the hydroelectric source, the CFP can be reduced by up to 24%, thus providing 11.20 kg CO2-Eq/FU with respect to the Fenton process with 20.74 kg CO2-Eq/FU.
Regarding the reagents necessary for the development of the EF-N and F-N processes, their contribution to the CFP represented 8.65% and 88.63%, respectively (1.28 kg CO2-Eq/FU vs. 18.38 kg CO2-Eq/FU). Note that the EF-N process requires significantly lower quantity of reagents, compared to F-N. The latter involves high doses of H2O2, FeSO4 and slaked lime (necessary for the neutralization stage). In the traditional F-N arrangement, a high amount of H2O2 required for its efficient operation (7.36 kg H2O2/FU, 50% w/w) in turn implies a greater output to CO2-Eq emissions, reaching 56.75% (11.77 kg CO2-Eq/FU) of the total CFP (Fig. 3b). Although H2O2 is considered a non-toxic compound, its industrial production, based on the catalytic oxidation of anthraquinone (ca. 95%), contributes significantly to the CFP. This is due to its: (1) high energy requirements; (2) use of organic solvents; (3) waste generation; (4) production stages involving high temperatures and pressures; (5) important investment in equipment for H2O2 purification; (6) difficulties in management associated with its transportation, storage, and handling, in high concentrations [84, 85]; and (7) high carbon footprint by itself (1.48 kg CO2-Eq/kg H2O2 produced, GWP-100), among others. Another reagent required in the F-N process with a high impact on the CFP was the slaked lime (24.04% of the total CFP, 4.99 kg CO2-Eq/FU). This was used in the neutralization stage to guarantee compliance with the maximum permissible discharge limits, in terms of pH, in accordance with current Colombian environmental legislation. Its significant contribution to the CFP is mainly due to the considerable amount necessary to neutralize the treated effluent (4.63 kg slaked lime/FU). At an industrial level, the production of this substance involves a series of stages that range from limestone extraction, crushing, washing and drying, selection of different limestone fractions, and calcination (temperatures > 1000 °C), to final production of hydrated lime Ca(OH)2. In environmental terms, the production of quicklime (CaO, slaked lime precursor) has a CFP of 1.2 kg CO2-Eq/kg CaO and that of slaked lime of 0.94 kg CO2-Eq/kg Ca(OH)2. This high environmental load is mainly related to the following: (1) the calcination stage of CaCO3 to obtain CaO and its subsequent slaking to reach the Ca(OH)2; two-thirds of CO2-Eq emissions are generated during this production stage; and (2) the use of fossil fuels in calcination kilns, the transportation of different materials, and the production of electricity for the operation of the plant (high energy demand, 3–9 MJ/kg CaO, 90% of the total energy consumption of the process) [86].
On the other hand, in the EF-N process, NaOH (the reagent with the greatest contribution in the CFP) represents 7.59% of the total CFP (1.12 kg CO2-Eq/FU) and 87.67% of the CFP associated with the reagents (Fig. 3a). In this process, NaOH was used as a neutralizing agent for the treated effluent to achieve the pH value according to the requirements of its discharge to surface water bodies. The required amount of NaOH was much lower (0.79 kg NaOH 50%/FU) than that of slaked lime (4.63 kg Ca(OH)2/FU) used in the F-N process. Although the CO2-Eq emissions related to NaOH production are slightly higher (1.32 kg CO2-Eq/kg NaOH in solution, 50 v/v %) than those from slaked lime production, the lower amount required for neutralization reduced the CFP associated with the consumption of reagents. Note that other reagents necessary for development of the EF-N process (H2SO4, FeSO4 and NaCl) only represent 1% of the total CFP.
Another important aspect involved in the operation of the sequential processes, evaluated in this study, is the acidification of the wastewater to be treated (2.8 < pH < 3.0). This to guarantee adequate conditions for the Fenton reaction. Based on the LCA, the contribution of H2SO4 in the total CFP denotes less than 0.5%. Finally, as seen in Fig. 3, the consumption of FeSO4 does not represent a limitation in the environmental performance of both sequential processes (F-N: 7.62%, 1.58 kg CO2-Eq/FU vs. EF-N: 0.54%, 0.08 kg CO2-Eq/FU) due to the low concentrations required, especially in the EF-N process.
In the infrastructure stage, the CFP is not significant for both processes (ca. 1%) and therefore does not represent an environmental problem in their performance. Likewise, the transport of materials and reagents is not substantial in the total CFP (less than 10%). However, this has a greater impact on the CFP in the case of the F-N process (1.28 kg CO2-Eq/FU), due to the number and quantities of reactants that need to be supplied (mainly H2O2, FeSO4 and slaked lime), compared to EF-N (0.064 kg CO2-Eq/FU).
The ReCiPe-2016 method at the midpoint level
The IPCC-2013 method allowed evaluating the performance of the F-N and EF-N processes, in terms of CO2-Eq emissions into the atmosphere. However, the assessment of additional environmental impact categories, through the ReCiPe-2016 method at the midpoint level, allows to obtain a more complete vision of the life cycle and its potential environmental hotspots. Note that the F-N process has a greater incidence in 14 of the 18 impact categories analyzed (Fig. 4).
Relative impact of sequential processes, EF-N and F-N, according to the ReCiPe-2016 method at the midpoint level. The acronyms included in the figure are defined in Table 2
Better environmental performance was estimated for the EF-N process. This is mainly due to the low consumption of reagents (FeSO4; H2O2 is not required since it is generated in situ) and auxiliary chemicals (H2SO4 and NaOH for the acidification and neutralization, respectively). Compared to the F-N process, the EF-N presented a notable relative impact in the IRP, FEP, PMFP, and TAP categories (Fig. 4). The IRP category is related to the emission of radionuclides into the environment, which can affect human health (increasing development of cancer, serious hereditary effects and damage to DNA molecules). The production of electricity from fossil fuels in thermal power plants (i.e., burning coal) is one of the activities that can impact in this category [78]. Thus, for the EF-N process, due to its higher electricity consumption, the relative impact in this category was more significant (43.82% greater than for the F-N). In the case of FEP category, the impact on water bodies is related to the discharge of nutrients (i.e., nitrogen, phosphorus) that implies eutrophication processes. They can endanger ecosystems and cause an increase in the rates of disappearance of autotrophic and heterotrophic species. For the EF-N process, the relative impact in this category was 36.46% more significant than for the F-N.
On the other hand, the lower relative impact of the EF-N process, between 9.39 and 81.37% compared to F-N, in all other categories stands out. This represents a positive aspect for its possible large-scale application. Some impact categories, like TETP, FETP, METP, HTPc, and HTPnc (associated with toxic potentials), presented the lowest contributions (Fig. 4). They are related to the emission of chemical substances that can generate persistent effects in organisms, the bioaccumulation of different type of substances in the human food chain, and even the toxic effect [78]. Their impact is noticeable in the F-N process due to the large quantity of reagents required for its operation, especially H2O2 and slaked lime. Figure 5 shows the relative impact of the most harmful stages during the considered sequential processes.
Relative impact of the most harmful stages in F-N (a) and EF-N (b), during their life cycle assessment according to the ReCiPe-2016 method at the midpoint level. The acronyms included in the figure are defined in Table 2
For the F-N, the reagent consumption stage represents contributions that vary between 47.83% and 96.86% in all categories (Fig. 5a), being mainly the use of H2O2 (58.90–95.57%), FeSO4 (1.46–30.49%), and slaked lime (1.20–26.68%) (Supplementary Material Figure S1a). As detailed in Sect. 3.1, the production of H2O2 involves an environmentally demanding industrial process. Its high consumption of electricity (1200 kWh/kg H2O2) and fossil fuels (oil:, 100 kWh/kg H2O2; coal and natural gas, 200 kWh/kg H2O2) [87] generate a series of emissions that affect the environmental footprint. On the other hand, the high requirement of FeSO4, to obtain Fe2+, impacts secondarily the consumption of reagents, despite the non-toxic nature of these ions. The second stage that has a notable influence is the transport of materials, reagents, and solid waste (0.54–29.68%, especially the transport of reagents, due to the high quantity required: 19.72 kg of reagents/FU). The category in which this stage has the greatest impact is land use (LOP: 29.68%), especially land transportation, which causes damage to ecosystems due to the loss of vegetation cover, alteration of the landscape, fragmentation of habitats, changes in land use, and loss of biodiversity, among others [78, 88]. Moreover, high production of sludge during the neutralization stage represents relative impacts that vary between 0.17 and 20.52% (Fig. 5a). Similarly to the CFP estimated using the IPCC-2013 method, the infrastructure (0.15–3.95%) and electricity (0.40–6.35%) stages have relative impacts of less than 10%, so they are not critical in the F-N process. For this reason, their contributions cannot be observed in Fig. 5a.
Two stages dominate the environmental performance of the EF-N process in all impact categories analyzed: electricity (44.67–92.24%) and reagents (7.49–37.33%) (Fig. 5b). Regarding electricity, its high impact is due to the fact that EF is highly demanding in terms of energy consumption. In fact, this represents the main drawback of its potential large-scale application from an environmental point of view. This is mostly due to the requirement for voltage transformation (Supplementary Material Fig. S2) and the subsequent emission of substances of environmental concern (SF6, O3, NOx) and consumption of non-renewable materials for its infrastructure [83]. These results are in agreement with those reported by Magdy et al. [56]. They evaluated the environmental footprint of different processes for the removal of phenol, including an Electro-Fenton. Of the 10 impact categories evaluated, the electricity consumption (especially due to the electrolysis process) had the greatest contribution in 9 of them, with approximate relative impacts between 70 and 95%.
In the reagent stage, although its consumption is low in this process, the neutralization with NaOH represents the greatest contribution in all categories (Supplementary Material Fig. S1b). The production of this reagent requires high energy consumption that varies between 2500 and 3000 kWh/ton NaOH and the emission of substances of environmental interest such as CH4, hydrofluorocarbons, and NOx, among others [89]. However, compared to the slaked lime used in the F-N process, NaOH has a lower impact since lower quantities are required to neutralize the residual water. The remaining stages of the EF-N process (infrastructure, 0.17–16.94%; transportation, 0.04–6.81%; residuals, 0.01–1.42%) are not significant due to the low quantity of reagents that require transportation (1.69 kg of reagents/FU) and the low sludge generation (0.21 kg/FU), so their contributions are not appreciated in Fig. 5b.
The ReCiPe-2016 method at the endpoint level
The ReCiPe-2016 method at the midpoint level allowed evaluating the performance of the sequential processes (F-N and EF-N) based on 18 categories focused on specific environmental impacts, providing a global perspective on the potential effects of these processes. An additional analysis of the LCA involves the application of the ReCiPe-2016 method at the endpoint level focused on three areas of protection: damage to human health (HH, unit: year), damage to ecosystem quality (ED, unit: species per year), and damage to resource availability (RA, unit: US dollar). These two approaches of the ReCiPe-2016 method, at the midpoint level and at the endpoint level, are complementary to each other in the interpretation of LCA. ReCiPe-2016 at the midpoint level presents low uncertainty. However, due to the number of impact categories involved, the results can be difficult to interpret and do not provide information regarding possible damages or final effects. On the other hand, ReCiPe-2016 at the endpoint level, although it presents greater uncertainty, provides information on the environmental relevance of aspects of special interest, which is easy to interpret [78].
The results of the application of ReCiPe-2016 method at the endpoint level showed that the EF-N process presents a lower contribution in the three impact categories, compared to the F-N (Fig. 6). Specifically, a reduction in impact of approximately 20% for the HH and ED categories and 55% in the case of the RA is observed.
Of the three areas evaluated, in the EF-N process two items dominate presenting more than 90% of incidence in the global impact: electricity (HH, 88.16%; ED, 88.75%; RA, 86.81%) and NaOH (HH, 7.62%; ED, 7.10%; RA: 6.66%) (Fig. 7a). Similar results were obtained at the midpoint level, where the PMFP (54.86%) and GWP (33.12%) categories presented the greatest potential impact on human health. These high contributions are due to the consumption of electricity originated from thermal energy sources such as coal (thermal efficiency of coal plants = 44%) and the voltage transformation required for its use. Furthermore, the production of electricity involves the use of various materials and energy that generate emissions of organic and inorganic substances and metals into the air (i.e., dioxins and furans, hydrocarbons, volatile organic compounds, CH4, CO2, CO, NOx, SOx, NH3, PM10, PM2.5, Al, As, Cd, Co, Cu, Pb, etc.) and contribute significantly to the PMFP and GWP categories [90]. On the other hand, the GWP (56.03%) and TAP (21.20%) categories have the highest contributions to ecosystem quality (ED). Terrestrial acidification (TAP) is caused by the deposition of inorganic substances in the soil, especially NOx, NH3, SO2, sulfates, and phosphates. These affect the growth of plants due to soil acidity conditions above the optimal level for normal development [78, 91]. Finally, resource availability (RA) is negatively affected by the FFP category (99.63%), given the high energy requirement of the EF-N process, which contributes to the scarcity of resources such as coal and natural gas.
On the other hand, the F-N process has three items that add up to more than 80% of incidence in the global impact: H2O2 (HH, 62.92%; ED, 60.74%; RA, 65.39%), slaked lime (HH, 12.36%; ED, 17.54%; RA, 14.49%), and transportation (HH, 7.06%; ED, 8.15%; RA, 11.17%) (Fig. 7b). In the case of H2O2 and slaked lime, the environmental performance is related to their production that involve high energy consumption, fossil fuels and gaseous emissions [84, 86]. In turn, the impact categories at the midpoint level that had the greatest contribution to the HH category were as follows: GWP (38.40%) and PMFP (37.55%). This indicates that emissions of greenhouse gases, particulate matter (PM2.5–PM10), and precursor gases of PM2.5 (SO2, NOx, and NH3, among others) are associated with damage to human health due to the increase in global temperature and respiratory diseases [78]. Similarly, in the ED area, the GWP category has a relative impact of 63.4%. Changes in temperature due to the emission of greenhouse gases can negatively impact ecosystem processes, the distribution and diversity of species, and food security and can cause forest fires and droughts, among other effects [92]. Finally, in the RA area, the scarcity of fossil resources (FFP), necessary for the production of reagents (H2O2 and slaked lime, mainly), has an impact of 99.26% on the depletion of these incomes.
Environmental footprint of Fenton-based chemical and electrochemical processes
Table 3 presents a comparison of the LCA results obtained in this study with some relevant ones reported in the literature for Fenton-based processes in the treatment of different types of synthetic and raw (industrial) effluents. Comparison of LCA-containing contributions is not a simple task due to differences in the definition of objectives and scope, the LCA assessment methods used (i.e., IPCC-2013, ReCiPe, CML baseline), the approach of LCA (i.e., cradle to gate, cradle to grave, gate to gate), treatment process, reactor type, effluent characteristics, etc. [63]. Most of these studies included within the limits of the system stages such as the production of reagents, electricity consumption, and construction materials. Only a few of them took into account phases such as sludge disposal, transportation of materials, and CO2 emissions from mineralization which implies an abbreviated estimate of the environmental footprint. On the other hand, when implementing an LCA study, depending on the objectives and scope, several assumptions are made (i.e., useful life of the wastewater treatment plants, location, mode of operation, operation stages, electricity generation mix, losses in the process, and airborne emissions, among others). Therefore, even when effluents have similar characteristics, LCA results can be substantially variable. As shown in Table 3, the lowest CFP values and operating costs are primarily associated with synthetic effluents with individual target contaminants (i.e., dyes, antibiotics). However, the complexity of raw wastewater significantly affects the total CFP, which could be attributed to the increase in reagent consumption for treatment processes.
Despite the different objectives and scopes, it can be highlighted that these LCA studies allow the identification of the main environmental critical points that must be addressed to improve their sustainability for future large-scale applications. As detailed in previous sections, the environmental impact of the Fenton process depends largely on the reagents used (mainly H2O2 and Ca(OH)2) and electricity consumption. In the latter case, its impact varies depending on the production source involved (i.e., fossil fuels or renewable energies). For example, Ioannou-Ttofa et al. [93] applied solar photo-Fenton using a CPC (Compound Parabolic Collector) type reactor for the secondary treatment of urban effluents contaminated with antibiotics. The results showed that electricity consumption was responsible for 91.6% of the total CFP (8.7 kg CO2-Eq/FU) (it was considered that 92.5% of the electricity came from oil). The production of electricity from fossil fuels involves different emissions into the atmosphere that contribute significantly to the increase in the CFP of the treatment processes. Similarly, Foteinis et al. [94] evaluated the sustainability of solar photo-Fenton process, in an autonomous CPC plant, for the treatment of a pharmaceutical effluent. The necessary electricity was provided by photovoltaic panels. The CFP was 2.71 kg CO2-Eq/FU, with the consumption of reagents (mainly, H2O2) accounting for 92.4%, despite the positive effect on the CFP due to the use of renewable energy. Compared to the Fenton process, there are few LCA studies for the EF case. In this study, electricity consumption was identified as the main environmental hotspot, contributing 87% to the total CFP (14.74 kg CO2-Eq/FU), while the consumption of reagent represented less than 10%. These results agree with those reported in Magdy et al. [56], who evaluated the environmental impacts of an EF process. The CFP obtained (26.78 kg CO2-Eq/FU) was due to the electricity consumption (> 90%), especially that necessary for the electrolysis process.
Thus, it is possible to conclude that the environmental footprint of AOPs is highly variable. However, the application of LCA studies represents an adequate alternative to identify the stages that contribute most to the environmental impacts, allowing actors to make decisions to improve the sustainability of the wastewater treatment processes.
Conclusions
This study reports for the first time on the sustainability of raw textile wastewater treatment through two sequential Fenton-based processes, chemical and electrochemical, based on Life Cycle Assessment (LCA) approach. The CF-F-N and CF-EF-N were optimized at laboratory scale and compared through LCA, using the IPCC-2013 and ReCiPe-2016 midpoint and endpoint methods.
According to the IPCC-2013 method, the highest CO2 emissions relied on the ITWW primary treatment by coagulation-flocculation. This is due to the high amount of hazardous sludge generated and the technology necessary for its disposal (i.e., 16.89 kg CO2-Eq/FU for underground deposit in security cells or 47.52 kg CO2-Eq/FU for incineration) as well as the consumption of reagents required for the treatment (alum, 7.72 kg CO2-Eq/FU; slaked lime, 5.56 kg CO2-Eq/FU). To successfully address this challenge, future research can focus on strategies aimed at reducing the amount of sludge formed and the reagents involved (e.g., electrocoagulation). Regarding the sequential processes, the EF-N presented lower CFP than the F-N (14.74 kg CO2-Eq/FU vs. 20.74 kg CO2-Eq/FU). Electricity (87.02% of the total CFP) and reagents (88.63% of the total CFP) denoted the main environmental hotspot during the EF-N and F-N, respectively.
According to the ReCiPe-2016 method at the midpoint level, the EF-N has an inferior incidence in 14 of the 18 impact categories analyzed, compared to the F-N. This is the result of low consumption of reagents (FeSO4; H2O2 is not required since it is generated in situ) and auxiliary chemicals (H2SO4 and NaOH for the acidification and neutralization, respectively). Similar to the results of IPCC-2013 method, the electricity was found as main environmental hotspot of the EF-N. This mainly due to the electricity consumption and the necessity of voltage transformation that involves emissions of SF6, O3, and NOx, and implies loss of electricity and requires non-renewable materials for its operation. For further applications, this drawback can be overpassed by the use of renewable energy sources (i.e., photovoltaic systems). In addition, the results of the ReCiPe-2016 method at the endpoint level showed that the EF-N resulted in lower environmental load in all impact categories, especially in RA, due to the lower reagent consumption.
In particular, the sequential combination of CF-EF-N can successfully improve the textile wastewater treatment efficiency while reducing the environmental impact, compared to the CF-F-N. The EF-N can be considered as more environmentally sustainable technology compared to traditional F-N. Finally, the application of LCA allowed to identify the main environmental hotspots (electricity for EF-N and reagents for F-N) that need to be addressed before the development and scaling-up for industrial applications of both sequential processes, promoting the accomplishment of Sustainable Development Goals (6, Clean Water and Sanitation; 9, Industry, Innovation, and Infrastructure; and 12, Responsible Consumption and Production). The economic performance (11.91 USD/m3 for CF-EF-N vs. 13.66 USD/m3 for CF-F-N) and LCA demonstrated the competitiveness of the electrochemical sequential process compared to the chemical one.
References
Naciones Unidas – Impacto académco (2024) Sostenibilidad. https://www.un.org/es/impacto-academico/sostenibilidad. Accessed 22 Jun 2024
Atia NG, Bassily MA, Elamer AA (2020) Do life-cycle costing and assessment integration support decision-making towards sustainable development? J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.122056
Ribeiro JP, Sarinho L, Nunes MI (2024) Application of life cycle assessment to Fenton processes in wastewater treatment – a review. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2023.104692
Market analysis report (2024) Textile market size & trends. https://www.grandviewresearch.com/industry-analysis/textile-market. Accessed 22 Jun 2024
Departamento Administrativo Nacional de Estadística (DANE) (2023) Boletín Técnico Producto Interno Bruto (PIB). https://www.dane.gov.co/index.php/estadisticas-por-tema/cuentas-nacionales. Accessed 22 Jun 2024
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manag. https://doi.org/10.1016/j.jenvman.2016.07.090
Departamento Administrativo Nacional de Estadística (DANE) (2023) Encuesta Ambiental Industrial. https://www.dane.gov.co/index.php/estadisticas-por-tema/ambientales/encuesta-ambiental-industrial-eai. Accessed 22 Jun 2024
Samsami S, Mohamadi M, Sarrafzadeh MH, Rene ER, Firoozbahr M (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2020.05.034
Ahmad A, Mohd-Setapar SH, Chuong CS, Khatoon A, Wani WA, Kumar R, Rafatullah M (2015) Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. https://doi.org/10.1039/c4ra16959j
Madhav S, Ahamad A, Singh P, Mishra PK (2018) A review of textile industry: wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manag. https://doi.org/10.1002/tqem.21538
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2018.06.060
Jun LY, Yon LS, Mubarak NM, Bing CH, Pan S, Danquah MK, Abdullah EC, Khalid M (2019) An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.102961
Collivignarelli MC, Abbà A, Carnevale Miino M, Damiani S (2019) Treatments for color removal from wastewater: state of the art. J Environ Manag. https://doi.org/10.1016/j.jenvman.2018.11.094
Martinez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev. https://doi.org/10.1039/B517632H
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev. https://doi.org/10.1021/acs.chemrev.5b00361
Garcia-Segura S, Eiband MMSG, de Melo JV, Martínez-Huitle CA (2017) Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2017.07.047
Martínez-Huitle CA, Panizza M (2018) Electrochemical oxidation of organic pollutants for wastewater treatment. Curr Opin Electrochem. https://doi.org/10.1016/j.coelec.2018.07.010
Ganiyu SO, Martínez-Huitle CA, Oturan MA (2021) Electrochemical advanced oxidation processes for wastewater treatment: advances in formation and detection of reactive species and mechanisms. Curr Opin Electrochem. https://doi.org/10.1016/j.coelec.2020.100678
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2023) A critical review on latest innovations and future challenges of electrochemical technology for the abatement of organics in water. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2023.122430
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review Appl Catal B: Environ. https://doi.org/10.1016/j.apcatb.2014.11.016
Manenti DR, Soares PA, Módenes AN, Espinoza-Quiñones FR, Boaventura RAR, Bergamasco R, Vilar VJP (2015) Insights into solar photo-Fenton process using iron(III)-organic ligand complexes applied to real textile wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2014.12.077
Doumic LI, Soares PA, Ayude MA, Cassanello M, Boaventura RAR, Vilar VJP (2015) Enhancement of a solar photo-Fenton reaction by using ferrioxalate complexes for the treatment of a synthetic cotton-textile dyeing wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2015.04.074
Chavaco LC, Arcos CA, Prato-Garcia D (2017) Decolorization of reactive dyes in solar pond reactors: perspectives and challenges for the textile industry. J Environ Manag. https://doi.org/10.1016/j.jenvman.2017.04.077
Hien SA, Trellu C, Oturan N, Assémian AS, Briton BGH, Drogui P, Adouby K, Oturan MA (2022) Comparison of homogeneous and heterogeneous electrochemical advanced oxidation processes for treatment of textile industry wastewater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2022.129326
Solano AMS, de Araújo CKC, de Melo JV, Peralta-Hernandez JM, da Silva DR, Martínez-Huitle CA (2013) Decontamination of real textile industrial effluent by strong oxidant species electrogenerated on diamond electrode: viability and disadvantages of this electrochemical technology. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2012.10.023
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2014.09.010
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J. https://doi.org/10.1016/j.cej.2017.11.059
Ramírez-Díaz RC, Prato-Garcia D (2021) Can thermal intensification be considered a sustainable way for greening Fenton processes? J Environ Manag. https://doi.org/10.1016/j.jenvman.2021.112551
Dobrosz-Gómez I, Quintero-Arias JD, Gómez-García MÁ (2024) Fenton advanced oxidation process for the treatment of industrial textile wastewater highly polluted with acid-black 194 dye. Case Stud Chem Environ Eng. https://doi.org/10.1016/j.cscee.2024.100672
Ministerio de Ambiente y Desarrollo Sostenible (MADS) (2015) Resolución 0631 de 2015. https://www.minambiente.gov.co/documento-normativa/resolucion-631-de-2015/. Accessed 22 Jun 2024
Ministerio de Ambiente y Desarrollo Sostenible (MADS) (2021) Resolución 1256 de 2021. https://www.minambiente.gov.co/documento-normativa/resolucion-1256-de-2021/. Accessed 22 Jun 2024
Zhou M, Oturan M, Sirés I (2018) Electro-Fenton process. Springer Nature Singapore Pte Ltd., New Trends and Scale-Up. https://doi.org/10.1007/978-981-10-6406-7
Feijoo S, Estévez S, Kamali M, Dewil R, Moreira MT (2023) Scale-up modelling and life cycle assessment of electrochemical oxidation in wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2022.140627
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem Rev. https://doi.org/10.1021/cr900136
Peralta-Hernández JM, Martínez-Huitle CA, Guzmán-Mar JL, Hernández-Ramírez A (2009) Recent advances in the application of electro-Fenton and photoelectro-Fenton process for removal of synthetic dyes in wastewater treatment. J Environ Eng Manag 19:257–265
Florenza X, Solano AMS, Centellas F, Martínez-Huitle CA, Brillas E, Garcia-Segura S (2014) Degradation of the azo dye Acid Red 1 by anodic oxidation and indirect electrochemical processes based on Fenton’s reaction chemistry. Relationship between decolorization mineralization and products. Electrochimica Acta. https://doi.org/10.1016/j.electacta.2014.07.117
Meriç S, Lofrano G, Belgiorno V (2005) Treatment of reactive dyes and textile finishing wastewater using Fenton’s oxidation for reuse. Int J Environ Pollut. https://doi.org/10.1504/IJEP.2005.006865
He Z, Huang C, Wang Q, Jiang Z, Chen J, Song S (2011) Preparation of a praseodymium modified Ti/SnO2-Sb/PbO2 electrode and its application in the anodic degradation of the Azo Dye Acid Black 194. Int J Electrochem Sci. https://doi.org/10.1016/S1452-3981(23)18332-5
Ledakowicz S, Bilińska L, Żyłła R (2012) Application of Fenton’s reagent in the textile wastewater treatment under industrial conditions. Ecol Chem Eng. https://doi.org/10.2478/v10216-011-0013-z
Wang Z, Fang C, Megharaj M (2014) Characterization of iron-polyphenol nanoparticles synthesized by three plant extracts and their Fenton oxidation of azo dye. ACS Sustain Chem Eng. https://doi.org/10.1021/sc500021n
Patil AD, Raut PD (2014) Treatment of textile wastewater by Fenton’s process as a Advanced Oxidation Process. IOSR J Environ Sci, Toxicol Food Technol. https://doi.org/10.9790/2402-081032932
Munteanu G, Karakashkova P, Eliyas A (2021) Parameter optimization of a semi-batch water decontamination slurry photocatalytic reactor using Taguchi-Grey technique. Bulg Chem Commun. https://doi.org/10.34049/bcc.53.4.5442
Martínez-Huitle CA, Vieira dos Santos E, Medeiros de Araújo D, Panizza M (2012) Applicability of diamond electrode/anode to the electrochemical treatment of a real textile effluent. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2012.02.005
Eslami A, Moradi M, Ghanbari F, Mehdipour F (2013) Decolorization and COD removal from real textile wastewater by chemical and electrochemical Fenton processes: a comparative study. J Environ Health Sci Eng. https://doi.org/10.1186/2052-336X-11-31
Rodrigues CSD, Madeira LM, Boaventura RAR (2014) Decontamination of an industrial cotton dyeing wastewater by chemical and biological processes. Ind Eng Chem Res. https://doi.org/10.1021/ie402750p
GilPavas E, Dobrosz-Gómez I, Gómez-García MÁ (2020) Efficient treatment for textile wastewater through sequential electrocoagulation, electrochemical oxidation and adsorption processes: Optimization and toxicity assessment. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2020.114578
Gómez CA, Gómez-García MÁ, Dobrosz-Gómez I (2023) Analysis of the capacity of the Fenton process for the treatment of polluted wastewater from the leather dyeing industry. Sci World J. https://doi.org/10.1155/2023/4724606
López A, Pagano M, Volpe A, Di Pinto A (2004) Fenton’s pretreatment of mature landfill leachate. Chemosphere
Deng Y, Englehard JD (2006) Treatment of landfill leachate by the Fenton process. Water Res. https://doi.org/10.1016/j.watres.2006.08.009
Yadav A, Mukherji S, Garg A (2013) Removal of chemical oxygen demand and color from simulated textile wastewater using a combination of chemical/physicochemical processes. Ind Eng Chem Res. https://doi.org/10.1021/ie400855b
Manenti DR, Módenes AN, Soares PA, Espinoza-Quiñones FR, Boaventura RAR, Bergamasco R, Vilar VJP (2014) Assessment of a multistage system based on electrocoagulation, solar photo-Fenton and biological oxidation processes for real textile wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2014.04.096
GilPavas E, Dobrosz-Gómez I, Gómez-García MÁ (2019) Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.09.125
International Organization for Standardization (2006) ISO 14040:2006. Environmental management – life cycle assessment – principles and framework. https://www.iso.org/obp/ui/#iso:std:iso:14040:ed-2:v1:en. Accessed 22 Jun 2024
Mahmud R, Moni SM, High K, Carbajales-Dale M (2021) Integration of techno-economic analysis and life cycle assessment for sustainable process design – a review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.128247
Belalcázar-Saldarriaga A, Prato-Garcia D, Vasquez-Medrano R (2018) Photo-Fenton processes in raceway reactors: technical, economic, and environmental implications during treatment of colored wastewaters. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.02.058
Magdy M, Gar Alalm M, El-Etriby HK (2021) Comparative life cycle assessment of five chemical methods for removal of phenol and its transformation products. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.125923
Bisinella de Faria AB, Spérandio M, Ahmadi A, Tiruta-Barna L (2015) Evaluation of new alternatives in wastewater treatment plants based on dynamic modelling and life cycle assessment (DM-LCA). Water Res. https://doi.org/10.1016/j.watres.2015.06.048
Vlasopoulos N, Memon FA, Butler D, Murphy R (2006) Life cycle assessment of wastewater treatment technologies treating petroleum process waters. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2006.03.007
Arzate S, Pfister S, Oberschelp C, Sánchez-Pérez JA (2019) Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.07.378
Chatzisymeon E, Foteinis S, Mantzavinos D, Tsoutsos T (2013) Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J Clean Prod. https://doi.org/10.1016/j.jclepro.2013.05.013
Li Y, Zhang S, Zhang W, Xiong W, Ye Q, Hou X, Wang C, Wang P (2019) Life cycle assessment of advanced wastewater treatment processes: involving 126 pharmaceuticals and personal care products in life cycle inventory. J Environ Manag. https://doi.org/10.1016/j.jenvman.2019.01
Grisales CM, Salazar LM, Prato-Garcia D (2019) Treatment of synthetic dye baths by Fenton processes: evaluation of their environmental footprint through life cycle assessment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-2757-9
Ahangarnokolaei MA, Attarian P, Ayati B, Ganjidoust H, Rizzo L (2021) Life cycle assessment of sequential and simultaneous combination of electrocoagulation and ozonation for textile wastewater treatment. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106251
Nakhate PH, Moradiya KK, Patil HG, Marathe KV, Yadav GD (2020) Case study on sustainability of textile wastewater treatment plant based on lifecycle assessment approach. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118929
Dobrosz-Gómez I, Quintero-Arias JD, Gómez-García MÁ (2024) Coagulation-Flocculation - Fenton-Neutralization sequential process for the treatment of industrial effluent polluted with AB194 dye. Case Stud Chem Environ Eng. https://doi.org/10.1016/j.cscee.2024.100720
Salazar-Sogamoso (2024) Discoloration and mineralization of a textile industrial wastewater through an electro-Fenton process with in-situ H2O2 generation, Master's Thesis in Engineering – Environmental Engineering. Universidad Nacional de Colombia, Sede Manizales
Quintero-Arias JD, Gómez-García MÁ, Dobrosz-Gómez I (2023) The scope of alum coagulation-flocculation assisted by slaked lime for the treatment of industrial wastewater containing highly concentrated Acid Black 194 dye. Optimization, molecular weight distribution and toxicity analysis. Results in Engineering. Under revision
NORMA TÉCNICA NTC-ISO COLOMBIANA 5667-2:1995 (1995) Guía sobre técnicas de muestreo. https://www.academia.edu/33146907/NORMA_T%C3%89CNICA_NTC_ISO_COLOMBIANA_5667_2. Accessed 22 Jun 2024
NORMA TÉCNICA NTC-ISO COLOMBIANA 5667-3:2004 (2004) Calidad del agua. https://www.studocu.com/co/document/universidad-nacional-de-colombia/quimica-analitica/ntc-iso-5667-03-2004-directrices-para-la-preservacion-y-manejo-de-muestras/29728969. Accessed 22 Jun 2024
NORMA TÉCNICA NTC-ISO COLOMBIANA 5667-1:2010 (2010) Calidad del agua. Muestreo. https://tienda.icontec.org/gp-calidad-del-agua-muestreo-parte-1-directrices-para-el-diseno-de-programas-y-tecnicas-de-muestreo-ntc-iso5667-1-2010.html. Accessed 22 Jun 2024
Instituto de Hidrología, Meteorología y Estudios Ambientales - IDEAM (2015) Toma y preservación de muestras. http://www.ideam.gov.co/documents/14691/38158/Toma_Muestras_AguasResiduales.pdf/f5baddf0-7d86-4598-bebd-0e123479d428. Accessed 22 Jun 2024
ASTM D2035–19 (2019) Practice for coagulation-flocculation jar test of water ASTM International. https://doi.org/10.1520/D2035-19
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. Centennial Edition of American Public Health Association, Washington, USA
International Organization for Standardization ISO 14044:2006 (2006) Environmental management – Life cycle assessment – Requirements and guidelines. https://www.iso.org/obp/ui/#iso:std:iso:14044:ed-1:v1:en. Accessed 22 Jun 2024
Klöpffer W, Grahl B (2014) Life cycle assessment (LCA). A guide to best practice. Wiley-VCH, Weinheim. https://doi.org/10.1002/9783527655625
Unidad de Planeación Minero Energética – UPME (2023) Proyección de la demanda de energía eléctrica, potencia máxima y gas natural 2023 - 2037. https://www1.upme.gov.co/DemandayEficiencia. Accessed 22 Jun 2024
Wernet G, Bauer C, Steubing B, Reinhard J, Moreno-Ruiz E, Weidema B (2016) The ecoinvent database version 3 (part I): overview and methodology. Int J Life Cycle Assess. https://doi.org/10.1007/s11367-016-1087-8
Huijbregts M, Steinmann Z, Elshout P, Stam G, Verones F, Vieira M, Hollander A, Zijp M, van Zelm M (2017) ReCiPe2016: a harmonised life cycle impact assessment method at midpoint and endpoint level. Int J Life Cycle Assess. https://doi.org/10.1007/s11367-016-1246-y
Sebastiano R, Contiello N, Senatore S, Righetti PG, Citterio A (2012) Analysis of commercial Acid Black 194 and related dyes by micellar electrokinetic chromatography. Dye Pigment. https://doi.org/10.1016/j.dyepig.2011.12.014
Ministerio de Ambiente y Desarrollo Sostenible (MADS) (2015) Decreto 1076 de 2015. https://www.minambiente.gov.co/documento-normativa/decreto-1076-de-2015/. Accessed 22 Jun 2024
de Jesus JON, Medeiros DL, Esquerre KPO, Sahin O, de Araujo WC (2024) Water treatment with aluminum sulfate and tanin-based biocoagulant in an oil refinery: the technical, environmental, and economic performance. Sustainability. https://doi.org/10.3390/su16031191
Environmental Protection Agency (EPA) (2023) Sulfur hexafluoride (SF6) Basics. https://www.epa.gov/eps-partnership/sulfur-hexafluoride-sf6-basics. Accessed 22 Jun 2024
Arvesen A, Hauan IB, Bolsøy BM, Hertwich EG (2015) Life cycle assessment of transport of electricity via different voltage levels: a case study for Nord-Trøndelag county in Norway. Appl Energy. https://doi.org/10.1016/j.apenergy.2015.08.013
Campos-Martin JM, Blanco-Brieva G, Fierro JLG (2006) Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew Chem Int Ed. https://doi.org/10.1002/anie.200503779
Cordeiro-Junior PJM, Kronka MS, Goulart LA, Veríssimo NC, Mascaro LH, dos Santos MC, Bertazzoli R, Lanza MR (2020) Catalysis of oxygen reduction reaction for H2O2 electrogeneration: the impact of different conductive carbon matrices and their physicochemical properties. J Catal. https://doi.org/10.1016/j.jcat.2020.09.020
Laveglia A, Sambataro L, Ukrainczyk N, De Belie N, Koenders E (2022) Hydrated lime life-cycle assessment: current and future scenarios in four EU countries. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.133224
Samanta C (2008) Direct synthesis of hydrogen peroxide from hydrogen and oxygen: an overview of recent developments in the process. Appl Catal A Gen. https://doi.org/10.1016/j.apcata.2008.07.043
Prato-Garcia D, Robayo-Avendaño A (2019) Treatment of a synthetic colored effluent in raceway reactors: The role of operational conditions on the environmental performance of a photo-Fenton process. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134182
Garcia-Herrero I, Margallo M, Onandía R, Aldaco R, Irabien A (2017) Environmental challenges of the chlor-alkali production: seeking answers from a life cycle approach. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.10.202
Wang Y, Pan Z, Zhang W, Borhani TN, Li R, Zhang Z (2022) Life cycle assessment of combustion-based electricity generation technologies integrated with carbon capture and storage: a review. Environ Res. https://doi.org/10.1016/j.envres.2021.112219
Turconi R, Boldrin A, Astrup T (2013) Life cycle assessment (LCA) of electricity generation technologies: overview, comparability and limitations. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2013.08.013
IPCC (2023) Summary for policymakers in: climate change 2023: synthesis report contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change. https://doi.org/10.59327/IPCC/AR6-9789291691647
Ioannou-Ttofa L, Foteinis S, Chatzisymeon E, Michael-Kordatou I, Fatta-Kassinos D (2016) Life cycle assessment of solar-driven oxidation as a polishing step of secondary-treated urban effluents. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.5126
Foteinis S, Monteagudo JM, Durán A, Chatzisymeon E (2018) Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.08.277
Salazar LM, Grisales CM, Prato-García D (2019) How does intensification influence the operational and environmental performance of photo-Fenton processes at acidic and circumneutral pH. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-2388-1
Conde J, Abelleira S, Estévez S, González-Rodríguez J, Feijoo G, Moreira MT (2023) Improving the sustainability of heterogeneous Fenton-based methods for micropollutant abatement by electrochemical coupling. J Environ Manag. https://doi.org/10.1016/j.jenvman.2023.117308
Pulido E, Santiago D, León E, Vaswani J, Herrera-Melián JA (2023) Treatment of laundry wastewater by different processes: optimization and life cycle assessment. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2023.109302
Zou M, Wei J, Qian Y, Xu Y, Fang Z, Yang X, Wang Z (2024) Life cycle assessment of homogeneous Fenton process as pretreatment for refractory pharmaceutical wastewater. Front Chem Sci Eng. https://doi.org/10.1007/s11705-024-2408-2
Rodríguez R, Espada JJ, Pariente MI, Melero JA, Martínez F, Molina R (2016) Comparative life cycle assessment (LCA) study of heterogeneous and homogenous Fenton processes for the treatment of pharmaceutical wastewater. J Clean Prod. https://doi.org/10.1016/j.jclepro.2016.02.064
Chai Y, Chen X, Wang Y, Guo X, Zhang R, Wei H, Jin H, Li Z, Ma L (2023) Environmental and economic assessment of advanced oxidation for the treatment of unsymmetrical dimethylhydrazine wastewater from a life cycle perspective. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2023.162264
Funding
Open Access funding provided by Colombia Consortium This research was funded by the Universidad Nacional de Colombia (Convocatoria para el Fortalecimiento de la Investigación, Creación, e Innovación Articulado con la Formación en la Universidad Nacional de Colombia 2020–2021: Proyectos: HERMES-51167, HERMES-51225 and HERMES-55259) as well as MINCIENCIAS (Convocatoria 852–2019, Proyecto: 202010034716, Contrato: 172–2021, HERMES-46681).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salazar-Sogamoso, L.M., Gómez-García, MÁ. & Dobrosz-Gómez, I. Comparative life cycle assessment of sequential chemical and electrochemical processes for the treatment of industrial textile wastewater. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05976-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05976-6