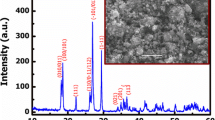
Abstract
Even though Pd-H system has been known since the nineteenth century, it is still of interest to scientists. Pd thin film electrode (called as a Limited Volume Electrode – LVE) is able to electrosorb hydrogen form aqueous (acid, base) and non-aqueous electrolytes (e.g. ionic liquids). Therefore Pd-H is an irreplaceable model system for the study of hydrogen electrosorption in different media. The paper presents: (1) short overview of the study of hydrogen sorption in Pd, (2) the detailed description of the electrochemical measurement methods and (3) the equations for the determination of hydrogen electrosorption charges needed to hydrogen electrosorption isotherms creation. The presented approach can be applied for laboratory work. The results show that Pd-LVE can be effectively used as a model system before the studies of multicomponent hydrogen storage alloys (e.g. AB5 alloys).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
A brief view of the study of hydrogen sorption in Pd
Interest in Pd-H system began in the 1930 when Sieverts proposed Pd-H phase diagram [1]. In the second half of the nineteenth century the results of research on palladium and its alloys were published by Graham [2]. Initially, the experiments, conducted by Schuldiner and Hoare [3], Frumkin and co-workers [4] as well as Lewis and Flanagan [5,6,7,8,9,10,11,12,13], mostly referred to the gas phase. The relative electrical resistance R/R0 has been very often used to measure the amount of absorbed hydrogen. Studies conducted by Lewis and Flanagan [5] show a very good agreement between the independently conducted measurements of electrical resistance and the atomic ratio of hydrogen to palladium: H/Pd. The relative electrical resistance increases linearly with the increase in the amount of hydrogen to the H/Pd value of about 0.55 (Fig. 1a) [8]. The Pd-H system (hydrogen absorbed from the gas phase) is presented in the literature in the form of isotherms characterizing the dependence of the absorbed hydrogen concentration on pressure (expressed as the hydrogen to palladium atomic ratio – H/Pd; Fig. 1b).
In Fig. 1b violet area delimits the two-phase region of the coexistence of the α and β phases of the absorbed hydrogen. In the presented case, the temperature of about 300 °C corresponds to the critical point, beyond which the two-phase area disappears. The pressure-composition (p–c) isotherms have a characteristic ‘S’ shape, in which three areas can be distinguished, corresponding to the existence of various forms of absorbed hydrogen. Flesh-color area (Fig. 1b) corresponds to a solid solution (α phase) of hydrogen in palladium, violet area can be attributed to the coexistence of α and β phases (absorption = phase transition α → β; desorption = phase transition β → α), blue area is the formation of pure β phase (non-stoichiometric palladium hydride).
The process of phase segregation of the absorbed hydrogen in Pd wire (solid) proposed by Flanagan and Lewis [6] is shown schematically in Fig. 2. When the atomic ratio of hydrogen to metal in the sample is close to 0.05, the maximum concentration of hydrogen in the α phase dominates. Starting from the surface phase, the concentration begins to increase and reaches only the minimum concentration at the surface of the sample in the β phase (Fig. 2a). The concentration profile "shifts" towards the depth of the sample until, at H/M≈0.5, almost the entire sample is saturated with hydrogen with a concentration higher than the minimum concentration of hydrogen in the β phase (Fig. 2b). Partial hydrogen oxidation causes the increase of the electrode potential which results from the decrease in the amount of absorbed hydrogen back to a concentration close to βmin (Fig. 2c).
Phase segregation diagram; a initial formation of the β-phase, surface concentration ≈ βmin; b almost complete conversion to the β phase; surface concentration > βmin; c conversion of the β phase after partial anodic oxidation of hydrogen; surface concentration ≈ βmin; based on [6]
Palladium easily absorbs hydrogen not only from the gas phase, but also electrochemically. Figure 3 shows curves characteristic for electrochemical measurements of the dependence of the amount of hydrogen absorbed on the sorption potential. On the presented curves, three characteristic areas, associated with the presence of various forms of absorbed hydrogen, can be distinguished. The first plateau at the higher potential values corresponds to the hydrogen adsorbed, trapped in the voids and the hydrogen absorbed in the α-phase (solid solution of hydrogen in palladium). In this region, the logarithm of H/M is directly proportional to the electrode potential, which is consistent with Sievert's equation relating to the gas phase [12]. In the electrochemical absorption the potential (above the hydrogen evolution potential) corresponds to the equilibrium hydrogen pressure in gas-phase experiments (higher pressure – lower potential) [5, 6]. Then, there is a significant jump in the amount of absorbed hydrogen in a narrow range of potential values, which is attributed to the α → β phase transition (absorption). The existence of another plateau is attributed to the formation of the β phase, i.e. non-stoichiometric hydride.
In the case of electrochemical measurements of Pd thin-film electrodes, the maximum H/Pd value that was obtained (for potentials close to the potentials of hydrogen evolution) is approx. 0.74. Under normal conditions, when Pd is in equilibrium with gaseous hydrogen, the H/Pd ratio is 0.69 [15]. In the case of electrodes with a thickness below 0.8 µm, a tendency to increased H/M was observed, even to a value of approx. 20% higher [16] than for electrodes with a thickness of approx. 1 µm. This is due to the presence of the hydrogen absorbed in the subsurface layer. In the case of thin samples subsurface layer contributes significantly to the total amount of hydrogen absorbed in Pd [16].
Tverdovskii and Vert et al. [17] were the first to use thin Pd layer for electrochemical research. In 1970s and 1980s hydrogen electrosorption phenomenon in Pd thin films was extensively studied by many researchers i.a. Bucur et al. [18,19,20,21], Chevillot et al. [22], Frazier et al. [23], Horkans [24], Nicolas et al. [25], Gossner et al. [26], Harris et al. [27]. The idea was continued in 1990s [28,29,30,31,32,33,34,35] and 2000s [36,37,38,39,40,41,42,43,44,45,46]. In 1999 Czerwinski introduced the concept of Pd limited volume electrode (Pd-LVE) for Pd electrodeposited on the gold substrate [16, 35, 47]. However, the term LVE can be extended to different kinds of electrodes fulfilling following requirements:
-
(a)
metal/alloy obtained by electrodeposition on substrate; it can also be a metal/alloy in the form of foil of a specific thickness or metal/alloy pressed in a net (net/substrate should be inert to hydrogen);
-
(b)
H absorption and adsorption currents of the same order enable studying simultaneously both bulk and surface reactions;
-
(c)
the process of diffusion of electroactive species (hydrogen) occurs inside a solid;
-
(d)
for hydrogen sorption measurements all hydrogen absorbing material should be immersed in the electrolyte.
Despite the passage of time the concept to use thin Pd layer or nanoparticles of Pd in hydrogen electrosorption experiment is still timely [48,49,50,51,52,53].
The electrochemical methods of hydrogen absorption and desorption measurements in Pd. What can be calculated from electrochemical data for Pd-H system?
Pd-LVE can be obtained by the electrodeposition of Pd on Au substate (Au wire) from aqueous galvanic bath containing Pd ions. Au is often utilized since it is important to use the substrate incapable to absorb hydrogen. Different potentiostatic or galvanostatic techniques can be utilized to electrodeposit Pd-LVE. In selection of electrodeposition conditions general principles should be taken into account: (a) 100% current efficiency can be obtained in potential range far from hydrogen and oxygen reduction (b) the geometry of deposition cell should support homogeneous distribution of deposit along the wire. As an example, the conditions of potentiostatic electrodeposition will be discussed below. In the electrodeposition experiment, chronoamperometry technique can be used to deposit the Pd at constant potential (e.g. ca. 0.39 V vs RHE) from chlorides-containing galvanic bath (e.g. 0.11 M PdCl2 in 1 M HCl) with the use of 3-electrode cell (see Fig. 4a): calomel or silver chloride electrode can be used as a reference electrode, Au wire as a working electrode and Pd spiral as an auxiliary electrode. Before the Pd electrodeposition Au substrate should be cleaned: (1) degreased with acetone (2) etched overnight in concentrated nitric acid (3) cycled in e.g. 0.5 M aqueous sulfuric acid solution (potential range of Au oxidation/reduction) to obtain repeatable CV behavior. Since the current efficiency of Pd electrodeposition (in the conditions described above) is close to 100% [54, 55], the mass (mPd) and the thickness (t) of the deposit can be calculated from Faraday’s law of electrolysis as follows:
where:
mPd – mass of electrodeposited Pd;
\({Q}_{{\text{red}}}^{{\text{Pd}}}\) – the charge consumed for the Pd ions reduction; calculated from the integration of CA curve;
M – molar mass of the Pd (106.42 g mol−1);
F – Faraday’s constant (96485.342 C mol−1).
where:
t – thickness of the Pd electrodeposit;
d – density of Pd (11.99 g cm−1);
S – geometric area of the Pd deposit, according to Fig. 5 and the following equation:
(assumptions done for ease of t estimation – as t compared to r is negligible it was omitted in above formula, for the same reason upper surface of deposit was omitted).
where:
r – the radius of Au wire (e.g. 0.25 mm).
h – the length of the Pd electrodeposit (in this study ca. 2 mm or 5 mm).
To obtain a layer of a certain thickness and length the charge of Pd ions reduction can be estimated as follows:
where V is the volume of the Pd electrodeposit:
and Sc is described as follows:
For example, to obtain 1 μm thick deposit (length 5 mm), the charge of ca. 0.17 C should be consumed for Pd ions reduction. Figure 6 presents SEM images of the electrodeposited Pd layer. Figure 6d shows good agreement between estimated and measured layer thickness.
After successful electrodeposition of Pd and before the main experiment of hydrogen sorption, Pd-LVE should be subjected to so called hydrogen pretreatment procedure (HPP) [55,56,57]. It is a series of cyclic voltammetry (CV) and chronoamperometry (CA) scans in the potential range involving alternating hydrogen absorption and desorption in Pd. HPP initial sorption/desorption cycles induce microstructural and lattice relaxation. HPP should be conducted until steady state of CA and CV is obtained. Hydrogen electrosorption experiment is conducted in 3-electrode cell presented in Fig. 4b. In the case of acidic, aqueous electrolyte containing sulfate anions mercury/mercurous sulfate electrode (Hg|Hg2SO4|0.5 M H2SO4) is used as a reference electrode, Pd electrodeposit as a working electrode and Pt gauze as an auxiliary electrode. In alkaline electrolyte mercury/mercurous oxide electrode Hg|HgO|0.5 M KOH is used as a reference electrode, whereas in ionic liquid-based electrolyte Ag wire is used as a pseudo-reference electrode. Figure 7 presents cyclic voltammetry behavior of Pd-LVE before and after HPP in three different electrolytes, two aqueous solutions: 0.5 M H2SO4, 0.5 M KOH and non-aqueous: the mixture of superacid with aprotic ionic liquid: 0.5 M HMS in EMIM-MS (0.5 M methanesulfonic acid in 1-ethyl-3-methylimidazolium methanesulfonate). Depending on the type of electrolyte, there is different proton donors available for the process of electrochemical sorption, according to the reaction equations below:
acid in water: | \({{\text{H}}}^{\oplus }+{{\text{e}}}^{\ominus }\to {{\text{H}}}_{{\text{ads}}}\to {{\text{H}}}_{{\text{abs}}}\) |
hydroxide in water: | \({{\text{H}}}_{2}{\text{O}}+{{\text{e}}}^{\ominus }\to {{\text{OH}}}^{\ominus }+{{\text{H}}}_{{\text{ads}}}\to {{\text{H}}}_{{\text{abs}}}\) |
acid in ionic liquid: | \({\text{RH}}+{{\text{e}}}^{\ominus }\to {{\text{R}}}^{\ominus }+{{\text{H}}}_{{\text{ads}}}\to {{\text{H}}}_{{\text{abs}}}\) |
It is well visible that HPP is crucial for continuing the experiment of electrochemical hydrogen sorption. HPP allows to increase the electrochemical reversibility of hydrogen sorption and enable to obtain such state of Pd-H system that does not change during further experiments. This procedure ensures the repeatability of the results. It is noteworthy that the process of hydrogen electrosorption is less reversible in IL-based media than in aqueous electrolytes even after application of HPP. Figure 7c and Fig. 7d indicate that proper selection of the scan rate is crucial in the studies of hydrogen electrosorption. In Fig. 7d it is visible that scan rate of 10 mVs−1 is too fast to obtain required stage of electrode preparation in IL-based electrolyte. HPP procedure applied with scan rate of 1 mVs−1 gives better results. This behavior is connected with relatively high viscosity and low ionic conductivity (see Table 1) of electrolytes based on the IL media. It is noteworthy that the value of appropriate scan rate should be also adjusted to the thickness of the studied electrode. The hydrogen saturation time increases with increasing Pd layer thickness, what entails the necessity of the scan rate reduction to obtain proper cyclic voltammetry characteristics [16, 35].
After HPP Pd electrode can be subjected to the main hydrogen electrosorption experiment. It involves three procedures: (1) hydrogen desorption measured by the means of chronoamperometry (CA), (2) hydrogen absorption measured by the means of CA, (3) hydrogen absorption measured by the means of cyclic voltammetry (CV). The latter two procedures should give similar results, however they both should be applied since CV gives ability to obtain hydrogen absorption isotherm (HAI) in wide range of potentials (also at the potential values of gaseous hydrogen generation).
In the case of hydrogen desorption measured chronoamperometrically (Fig. 8a), first of all the hydrogen is sorbed in Pd at − 0.7 V vs MSE (mercury/mercurous sulfate electrode) in acidic media (ca. − 0.02 V vs RHE) and then oxidized in different potential values (in the potential range ca. − 0.2/ − 0.68 V vs MSE (0.5/ − 0.003 V vs RHE); increment 5 mV near the phase transition). The amount of hydrogen expressed as hydrogen to palladium atomic ratio (H/Pd) can be calculated as follows:
where:
\({Q}_{{\text{ox}},\mathrm{ max}}^{{\text{H}}}\) – the maximum charge consumed for the hydrogen oxidation (at potential value of ca. 0.5 V vs. RHE); calculated from the integration of CA desorption curve;
\({Q}_{{\text{ox}}}^{{\text{H}}}\) – the charge consumed for the hydrogen oxidation at selected potential value; calculated from the integration of CA desorption curve;
In the case of hydrogen absorption measured chronoamperometrically (Fig. 8b), hydrogen is electrosorbed at different potential values (in the potential range of − 0.6 V to − 0.7 V vs MSE (0.08 V to − 0.02 V vs RHE). The attention should be paid on the time of absorption, since the phase transition is the slowest process, there can be observed the prolongation of the absorption time until the phase transition occurs. Then the time of absorption decreases. The experiment should be led until the background current is obtained. It ensures that all the hydrogen is removed from the Pd. The amount of hydrogen (H/Pd) can be calculated as follows:
where:
\({Q}_{{\text{red}}}^{{\text{H}}}\) – the charge consumed for the hydrogen reduction at selected potential value; calculated from the integration of CA desorption curve;
In the third procedure hydrogen absorption is measured with the use of cyclic voltammetry (Fig. 8c). In this procedure the amount of absorbed hydrogen is calculated from signal of hydrogen oxidation. Hydrogen absorption occurs at constant potential value (between − 0.45 V and − 0.8 V vs MSE (0.2 V/ − 0.12 V vs RHE)) and is oxidized by means of CV in the potential range from the absorption potential to the higher potential value (e.g. − 0.2 V vs MSE (0.5 V vs RHE)) where hydrogen is fully oxidized and the current drops to the background current. The amount of hydrogen (H/Pd) can be calculated as follows:
If the amount of hydrogen is calculated from electrochemical measurements, there are some additional (apart from absorbed hydrogen) contributions that should be considered in calculation of the total charge:
-
(A)
H2 evolution (H2 gas below 0 V vs RHE, dissolved H2 molecules above 0 V vs RHE) is the most important, especially at low cathodic limits.
-
(B)
Hydrogen adsorption. This contribution typically cannot be separated easily, as desorption of hydrogen from α-phase and from the surface occurs in the same potential interval.
-
(C)
Oxygen reduction in high potential limits. Concentration of oxygen under usual deaeration conditions (pure Ar, sufficiently long deaeration) can be decreased for an order of magnitude as compared to aerated solution.
Contributions (A) and (C) always increase cathodic charge and decrease anodic charge. However, Czerwinski and co-workers found that for Pd-LVE [35] contribution (A) do not affect the hydrogen oxidation charge. In the presented results only contribution (B) can affect slightly the overall charge, but the contribution of adsorbed hydrogen constitutes ca. 1% of the total charge. A significant effect from adsorbed hydrogen could be expected only for very rough and thin electrode (for detailed explanation see [58]). Quantitatively all these contributions remain minor only if the amount of Pd is high enough, and scan rate is not very slow. Each new experiment with lower amount of Pd requires special tests to check by-side contributions.
Electrochemical hydrogen absorption isotherm (HAI)
After the calculation of the amount of hydrogen H/Pd from the electrochemical signals registered in different potential values the dependence of H/Pd vs E can be plotted. Figure 9 presents the results obtained from the third (cyclic voltammetry) procedure described at the previous paragraph. The course of the isotherms corresponds to the process of α → β phase transition. In the case of aqueous solutions the shape of the isotherms resembles the one presented in Fig. 3. Moreover, hydrogen absorption isotherms for acidic and alkaline solutions overlap, showing the same value of the α → β phase transition potential (ca. 0.05 V vs RHE, the same as obtained from the gas phase measurement). For the IL-based media the course of isotherm in the two-phase region is different without abrupt change of the hydrogen capacity. It is worth noting that maximum hydrogen capacity is comparable regardless of the type of the electrolyte. This behavior can be ascribed mostly to the strong differences in viscosity and ionic conductivity in studied electrolytes (Table 1). The ionic conductivity of acidic aqueous solution is ca. 95 times higher than ionic conductivity of electrolyte based on ionic liquid. Whereas viscosity of studied ionic liquid is ca. 124 times higher than viscosity of acidic aqueous solution.
It should be underlined that equilibrium issue have to be discussed for the potential values below 0 V vs RHE and in the case of using cyclic voltammetry in determination of HAI. Every point created HAI was collected through preliminary saturation of Pd-LVE in sufficiently long time allowing for full saturation with hydrogen and then the hydrogen was oxidized by means of cyclic voltammetry with appropriately selected scan rate (for ILs-based electrolytes it was 1mVs−1). Moreover, very good consistency of the results has been observed in HAIs obtained by CA and CV measurements (second and third procedure). It should be also added that non-equilibrium behavior can be also a result of the slow phase transition process. The process of the phase transition in ILs-based media should be studied further since α → β phase transition potential should not be dependent on the proton donor – as it can be noticed form Fig. 9 the slope of the HAI differs for aqueous solutions and ILs-based media.
Why Pd-LVE is an irreplaceable model system to study hydrogen electrosorption in various electrolytes?
The use of Pd-LVE and application of the three described, electrochemical procedures successfully enables to obtain hydrogen electrosorption parameters in different electrolytes, even those with relatively high viscosity and low ionic conductivity. In the case of preliminary measurements of hydrogen electrosorption experiment in previously untested electrolyte it is advisable firstly to apply Pd-LVE before the use of a standard, multicomponent hydrogen absorbing material (eg. AB5 alloy). Due to its limited thickness (mostly up to 1 μm) electrochemical response of the system, regardless of the type of the electrolyte is really fast. Another advantage of the Pd-LVE compared to standard hydrogen absorbing material is similar values of hydrogen absorption and desorption potentials. Obtaining satisfactory parameters of hydrogen electrosorption experiment with Pd-LVE in some selected electrolytes pave the way to further experiments with more complicated, multicomponent standard hydrogen absorbing alloys.
Data availability
https://doi.org/10.18150/B7LLRP, RepOD.
References
Sieverts A, Zapf G (1935) Die Löslichkeit von Deuterium und von Wasserstoff in festem Palladium. Z Phys Chem 174:359
Graham T (1866) XVIII On the absorption and dialytic separation of gases by colloid septa. Philos Trans R Soc Lond 156:399–439. https://doi.org/10.1098/rstl.1866.0018
Schuldiner S, Hoare JP (1955) Note on the Potential of the α-Pd/H2 Electrode. J Chem Phys 23:1551–1552. https://doi.org/10.1063/1.1742361
Frumkin AN (1963) Advances in electrochemistry and electrochemical engineering. Interscience Pub, New York 3:307
Flanagan TB, Lewis FA (1959) Hydrogen absorption by palladium in aqueous solution. Trans Faraday Soc 55:1400–1408. https://doi.org/10.1039/TF9595501400
Flanagan TB, Lewis FA (1959) Electrode potentials of the palladium+ hydrogen system. Trans Faraday Soc 55:1409–1420. https://doi.org/10.1039/TF9595501409
Lewis FA (1960) The hydrides of palladium and palladium alloys. Platin Met Rev 4(4):132–137. https://technology.matthey.com/article/4/4/132-137/. Accessed 20 Sept 2023
Lewis FA (1961) The hydrides of palladium and palladium alloys. Platin Met Rev 5(1):21–25. https://technology.matthey.com/article/5/1/21-25/. Accessed 20 Sept 2023
Lewis FA (1982) The Palladium-Hydrogen System. A Survey of Hydride Formation and the Effects of Hydrogen Contained Within the Metal Lattices. Platin Met Rev 26(1):20–27. https://technology.matthey.com/article/26/1/20-27/. Accessed 20 Sept 2023
Lewis FA (1982) The Palladium-Hydrogen System. Part II of a Survey of Features. Platin Met Rev 26(2):70–78. https://technology.matthey.com/article/26/2/70-78/. Accessed 20 Sept 2023
Lewis FA (1982) The Palladium-Hydrogen System. Part III: Alloy Systems and Hydrogen Permeation. Platin Met Rev 26(3):121–128. https://technology.matthey.com/article/26/3/121-128/. Accessed 20 Sept 2023
Lewis FA (1994) Palladium-hydrogen system. Structures Near Phase Transition and Critical Points. Platin Met Rev 38(3):112–118. https://technology.matthey.com/article/38/3/112-118/. Accessed 20 Sept 2023
Lewis FA (1967) The palladium hydrogen system. Academic Press, London and New York
Dekura S, Kobayashi H, Kusada K, Kitagawa H (2019) Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal-Organic Framework Coating Effects. ChemPhysChem 20(10):1158–1176. https://doi.org/10.1002/cphc.201900109
Woods R (1976) Chemisorption at electrodes. In: Bard AJ (ed) Electroanalytical chemistry, vol 9. Marcel Dekker, New York, pp 2–162
Czerwiński A, Kiersztyn I, Grdeń M (2000) The study of hydrogen sorption in palladium limited volume electrodes (Pd-LVE): Part II. Basic solutions J Electroanal Chem 492(2):128–136. https://doi.org/10.1016/S0022-0728(00)00291-6
Sokolski DV (1962) Hydrogenation in solutions. Nauka, Alma-Ata
Bucur RV, Stoicovici L (1970) Hydrogen solubility in electrolytically deposited thin films of palladium. J Electroanal Chem Interfacial Electrochem 25(2):342–343. https://doi.org/10.1016/S0022-0728(70)80351-5
Bucur RV, Mecea V, Indrea E (1976) The mechanism of hydrogen sorption by thin palladium layers I. Desorption J Less-Common Met 49:147–158. https://doi.org/10.1016/0022-5088(76)90032-1
Bucur RV, Flanagan TB (1974) The Effect of the Absorption of Hydrogen and Deuterium on the Frequency of a Quartz-Palladium Resonator. Z Phys Chem NF 88(5–6):225–241. https://doi.org/10.1524/zpch.1974.88.5_6.225
Bucur RV, Mecea V (1980) Equilibrium and kinetic measurements on thin Pd-H layers. Surf Techn 11(5):305–322. https://doi.org/10.1016/0376-4583(80)90049-7
Chevillot JP, Farcy J, Hinnen C, Rousseau A (1975) Electrochemical study of hydrogen interaction with palladium and platinum. J Electroanal Chem Interfacial Electrochem 64(1):39–62. https://doi.org/10.1016/S0022-0728(75)80277-4
Frazier GA, Glosser R (1980) Characterization of thin films of the palladium-hydrogen system. J Less-Common Met 74(1):89–96. https://doi.org/10.1016/0022-5088(80)90077-6
Horkans J (1980) Film thickness effects on hydrogen sorption at palladium electrodes. J Electroanal Chem Interfacial Electrochem 106:245–249. https://doi.org/10.1016/S0022-0728(80)80171-9
Nicolas M, Raffy H, Dumoulin L, Burger JP (1987) Absorption of hydrogen in ultrathin palladium and palladium-based alloys. J Less-Common Met 130:61–67. https://doi.org/10.1016/0022-5088(87)90087-7
Gossner K, Mizera E (1981) Alloy formation at the deposition of palladium on gold at room temperature. J Electroanal Chem Interfacial Electrochem 125(2):359–366. https://doi.org/10.1016/S0022-0728(81)80354-3
Harris LA (1982) Thin palladium films on silicon and titanium. J Electrochem Soc 129(12):2689–2694. https://doi.org/10.1149/1.2123649
Rosamilia JM, Abys JA, Miller B (1991) Electrochemical hydrogen insertion into palladium and palladium-nickel thin films. Electrochim Acta 36(7):1203–1208. https://doi.org/10.1016/0013-4686(91)85110-S
Tateishi N, Yahikozawa K, Nishimura K, Suzuki M, Iwanaga Y, Watanabe M, Enami E, Matsuda Y, Takasu Y (1991) Electrochemical properties of ultra-fine palladium particles for adsorption and absorption of hydrogen in an aqueous HClO4 solution. Electrochim Acta 36(7):1235–1240. https://doi.org/10.1016/0013-4686(91)85114-M
Attard GA, Bannister A (1991) The electrochemical behaviour of irreversibly adsorbed palladium on Pt (111) in acid media. J Electroanal Chem Interfacial Electrochem 300(1–2):467–485. https://doi.org/10.1016/0022-0728(91)85411-H
Szpak S, Mosier-Boss PA, Scharber SR, Smith JJ (1992) Charging of the Pd/nH system: role of the interphase. J Electroanal Chem 337(1–2):147–163. https://doi.org/10.1016/0022-0728(92)80534-B
Cheek GT, O’Grady WE (1994) Measurement of H/D uptake characteristics at palladium using a quartz crystal microbalance. J Electroanal Chem 368(1–2):133–138. https://doi.org/10.1016/0022-0728(93)03079-5
Gräsjo L, Seo M (1990) Measurement of absorption of hydrogen and deuterium into palladium during electrolysis by a quartz crystal microbalance. J Electroanal Chem Interfacial Electrochem 296(1):233–239. https://doi.org/10.1016/0022-0728(90)87245-F
Yamamoto N, Ohsaka T, Terashima T, Oyama N (1990) In situ electrochemical quartz crystal microbalance studies of water electrolysis at a palladium cathode in acidic aqueous media. J Electroanal Chem Interfacial Electrochem 296(2):463–471. https://doi.org/10.1016/0022-0728(90)87266-M
Czerwiński A, Kiersztyn I, Grdeń M, Czapla J (1999) The study of hydrogen sorption in palladium limited volume electrodes (Pd-LVE): I. Acidic solutions J Electroanal Chem 471(2):190–195. https://doi.org/10.1016/S0022-0728(99)00276-4
Bartlett PN, Gollas B, Guerin S, Marwan J (2002) The preparation and characterisation of H1-e palladium films with a regular hexagonal nanostructure formed by electrochemical deposition from lyotropic liquid crystalline phases. Phys Chem Chem Phys 4(15):3835–3842. https://doi.org/10.1039/B201845D
Paillier J, Roué L (2005) Physical and analytical electrochemistry-nanostructured palladium thin films prepared by pulsed laser deposition-structural characterizations and hydrogen electrosorption properties. J Electrochem Soc 152:E1–E8. https://doi.org/10.1149/1.1825952
Łosiewicz B, Birry L, Lasia A (2007) Effect of adsorbed carbon monoxide on the kinetics of hydrogen electrosorption into palladium. J Electroanal Chem 611(1–2):26–34. https://doi.org/10.1016/j.jelechem.2007.07.025
Lasia A (2006) On the mechanism of the hydrogen absorption reaction. J Electroanal Chem 593(1–2):159–166. https://doi.org/10.1016/j.jelechem.2006.03.049
Birry L, Lasia A (2006) Effect of crystal violet on the kinetics of H sorption into Pd. Electrochim Acta 51(16):3356–3364. https://doi.org/10.1016/j.electacta.2005.09.034
Martin MH, Lasia A (2009) Hydrogen sorption in Pd monolayers in alkaline solution. Electrochim Acta 54(22):5292–5299. https://doi.org/10.1016/j.electacta.2009.01.051
Duncan H, Lasia A (2007) Mechanism of hydrogen adsorption/absorption at thin Pd layers on Au(1 1 1). Electrochim Acta 52(21):6195–6205. https://doi.org/10.1016/j.electacta.2007.03.068
Lebouin C, Olivier YS, Sibert E, Millet P, Maret M, Faure R (2009) Electrochemically elaborated palladium nanofilms on Pt(1 1 1): Characterization and hydrogen insertion study. J Electroanal Chem 626(1–2):59–65. https://doi.org/10.1016/j.jelechem.2008.11.005
Oliveira MCF (2006) A new approach to prepare highly loaded palladium. Electrochem Commun 8(4):647–652. https://doi.org/10.1016/j.elecom.2006.02.011
Bertoncello P, Peruffo M, Unwin PR (2007) Formation and evaluation of electrochemically-active ultra-thin palladium–Nafion nanocomposite films. Chemical Commun 16:1597–1599. https://doi.org/10.1039/B702537H
Rusanova MY, Grden M, Czerwinski A, Tsirlina GA, Petrii OA, Safonova TY (2001) Isotope effects in α-PdH (D) as an instrument for diagnosing bulk defects. J Solid State Electrochem 5:212–220. https://doi.org/10.1007/s100080000135
Łukaszewski M, Czerwiński A (2011) Application of Thin Layer of Metal Deposit for Easy Study on Hydrogen Electrosorption in Palladium. Chem Educator 16:138–142. http://www.chemeducator.org/bibs/0016001/16110138.htm. Accessed 20 Sept 2023
Pająk M, Hubkowska K, Czerwiński A (2022) Improved hydrogen sorption properties of Pd in protic and aprotic ionic liquids effected by superacid addition. J Alloys Compd 903:163853. https://doi.org/10.1016/j.jallcom.2022.163853
Hubkowska K, Pająk M, Soszko M, Czerwiński A (2023) The Electrochemical Behavior of Unmodified and Pd-NPs Modified AB5 Hydrogen Storage Alloy in Selected Protic and Aprotic Ionic Liquids (ILs): Towards ILs-Based Electrolytes for Ni-MH Batteries. Molecules 28(2):856. https://doi.org/10.3390/molecules28020856
Boateng E, van der Zalm J, Chen A (2021) Design and electrochemical study of three-dimensional expanded graphite and reduced graphene oxide nanocomposites decorated with Pd nanoparticles for hydrogen storage. J Phys Chem C 125(42):22970–22981. https://doi.org/10.1021/acs.jpcc.1c06158
Viola A, Chattot R, Martin V, Tsirlina G, Nelayah J, Drnec J, Maillard F (2023) Hydrogen Trapping in Palladium Nanoparticles Revealed by Electrochemical, X-ray Scattering, and Spectrometric Measurements. J Phys Chem C 127(36):17761–17769. https://doi.org/10.1021/acs.jpcc.3c04464
Moumaneix L, Rautakorpi A, Kallio T (2023) Interactions between Hydrogen and Palladium Nanoparticles: Resolving Adsorption and Absorption Contributions. ChemElectroChem 10(6):e202201109. https://doi.org/10.1002/celc.202201109
Scandurra A, Censabella M, Gulino A, Grimaldi MG, Ruffino F (2022) Electro-Sorption of Hydrogen by Platinum, Palladium and Bimetallic Pt-Pd Nanoelectrode Arrays Synthesized by Pulsed Laser Ablation. Micromachines 13(6):963. https://doi.org/10.3390/mi13060963
Hubkowska K, Łukaszewski M, Czerwiński A (2018) Quartz crystal nanobalance measurements in electrocatalysis. Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, Elsevier, 402–412. https://doi.org/10.1016/B978-0-12-409547-2.13365-7
Pająk M, Hubkowska K, Czerwiński A (2019) Nitrate protic ionic liquids as electrolytes: Towards hydrogen sorption in Pd. Electrochim Acta 324:134851. https://doi.org/10.1016/j.electacta.2019.134851
Hubkowska K, Czerwiński A (2020) Tuning hydrogen sorption properties of Pd by its alloying with Ru, Rh, and Pt: the study of binary alloys in concentrated alkaline media. J Solid State Electrochem 24:3135–3143. https://doi.org/10.1007/s10008-020-04776-y
Pająk M, Hubkowska K, Czerwiński A (2018) The study of hydrogen sorption in palladium limited volume electrode from DEMA-TFO ionic liquid. J Electroanal Chem 825:73–76. https://doi.org/10.1016/j.jelechem.2018.08.014
Hubkowska K, Koss U, Łukaszewski M, Czerwiński A (2013) Hydrogen electrosorption into Pd-rich Pd-Ru alloys. J Electroanal Chem 704:10–18. https://doi.org/10.1016/j.jelechem.2013.06.004
Funding
This work was funded by National Science Centre (NCN, Poland), grant number 2021/41/B/ST5/04047.
Author information
Authors and Affiliations
Contributions
Katarzyna Hubkowska: Conceptualization, Writing – original draft, Visualization, Supervision. Małgorzata Pająk: Writing – original draft. Dorota Monikowska: Writing – original draft, Visualization. Andrzej Czerwiński: Writing – review & editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hubkowska, K., Pająk, M., Monikowska, D. et al. Pd-H as an irreplaceable model system for the study of hydrogen electrosorption in aqueous and non-aqueous electrolytes. J Solid State Electrochem 28, 1159–1169 (2024). https://doi.org/10.1007/s10008-024-05799-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-024-05799-5