Abstract
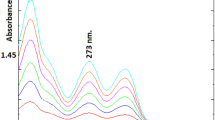
In this study, we introduce an innovative approach to electroanalysis that combines solvent extraction and chronocoulometry for the quantification of furosemide, a loop diuretic medication. The objective of this report is to provide students with a practical learning experience in the field of electroanalysis, enabling them to explore recent developments both theoretically and in the laboratory. The experimental procedure involves the extraction of furosemide from a pharmaceutical tablet using methanol, followed by the deposition of the extracted compound onto an electrode. Chronocoulometry is then employed to determine the quantity of furosemide. A 95% recovery rate and a close to 99% fit between theoretical and experimental substance amounts demonstrate the applicability of the method. This approach offers several didactic benefits for students: (i) It teaches the theory and application of chronocoulometry. (ii) It emphasizes the advantage of chronocoulometry as a calibration-free technique. (iii) It introduces students to the principles of electrochemical stripping techniques. (iv) It demonstrates that solid particles can be effectively studied using electroanalysis. Finally, (v) it provides a real-world application of electroanalysis in quantifying an organic compound.





Similar content being viewed by others
Change history
08 January 2024
Springer Nature’s version of this paper was updated: Author biographies and photos has been adjusted.
References
Domínguez I, Doménech-Carbó A (2015) Screening and authentication of tea varietiesbased onmicroextraction-assisted voltammetry of microparticles. Sens Act B 210:491–499. https://doi.org/10.1016/j.snb.2015.01.009
Doménech-Carbó A, Ibars AM, Prieto-Mossi J, Estrelles E, Scholz F, Cebrián-Torrejóna MM (2015) Electrochemistry-based chemotaxonomy in plants using the voltammetry of microparticles methodology. New J Chem 39:7421–7428. https://doi.org/10.1039/C5NJ01233C
Ortiz-Miranda AS, König P, Kahlert H, Scholz F, Osete-Cortina L, Doménech-Carbó MT, Doménech-Carbó A (2016) Voltammetric analysis of Pinus needles with physiological, phylogenetic, and forensic applications. Anal Bioanal Chem 408:4943–4952. https://doi.org/10.1007/s00216-016-9588-7
Muñiz-Calvo S, Guillamón JM, Domínguez I, Doménech-Carbó A (2017) Detecting and monitoring the production of melatonin and other related indole compounds in different saccharomyces strains by solid-state electrochemical techniques. Food Anal Methods 10:1408–1418. https://doi.org/10.1007/s12161-016-0699-8
Domingos da Silveira G, Di Turo F, Dias D, Fracassi da Silva JA (2020) Electrochemical analysis of organic compounds in solid-state: applications of voltammetry of immobilized microparticles in bioanalysis and cultural heritage science. J Solid State Electrochem 24:2633–2652. https://doi.org/10.1007/s10008-020-04720-0
Gulppi MA, Vejar N, Tamayo L, Azocar MI, Vera C, Silva C, Zagal JH, Scholz F, Páez MA (2014) Stripping voltammetry microprobe (SPV): a new approach in electroanalysis. Electrochem Commun 41:8–11. https://doi.org/10.1016/j.elecom.2014.01.012
Gulppi M, Pavez J, Zagal JH, Sancy M, Azocar M, Scholz F, Páez MA (2015) Stripping voltammetry microprobe (SPV): substantial improvements of the protocol. J Electroanal Chem 745:61–65. https://doi.org/10.1016/j.jelechem.2015.03.008
Scholz F, Meyer B (1998) Voltammetry of solid microparticles immobilized on electrode surfaces. Electroanal Chem 20
Scholz F, Schroeder U, Gulaboski R, Antonio DC (2015) Electrochemistry of immobilized particles and droplets 2nd Edition, Experiments with three phase electrodes. https://eprints.ugd.edu.mk/id/eprint/12083
Doménech-Carbó A, Labuda J, Scholz F (2012) Electroanalytical chemistry for the analysis of solids: characterization and classification (IUPAC Technical Report). Pure Appl Chem 85(3):609–631. https://doi.org/10.1351/PAC-REP-11-11-13
Tang N, Wang X, Du W, Zhang L, Xiang J, Wang S, Cheng P, Zhu L, Yin Q (2019) Conformational flexibility and crystallization: the case of furosemide. Crystal Growth Design 19:2050–2059. https://doi.org/10.1021/acs.cgd.8b01407
Kahlert H, Scholz F (2013) Acid-Base Diagrams Springer Berlin 978-3-642-37901-7 (Print) 978-3-642-37902-4 (eBook). https://doi.org/10.1007/978-3-642-37902-4
Baka E, Comer JEA, Takács-Novák K (2008) Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal 46:335–341. https://doi.org/10.1016/j.jpba.2007.10.030
Bukkitgar SD, Shetti NP (2016) Electrochemical oxidation of loop diuretic furosemide in aqueous acid medium and its analytical application. Cogent Chem 2(1):1152784. https://doi.org/10.1016/j.ejps.2009.04.009
Malode SJ, Abbar JC, Shetti NP, Nandibewoor ST (2012) Voltammetric oxidation and determination of loop diuretic furosemide at a multi-walled carbon nanotubes paste electrode. Electrochim Acta 60:95–101. https://doi.org/10.1016/j.electacta.2011.11.011
Shetti NP, Sampangi LV, Hegde RN, Nandibewoor ST (2009) Electrochemical oxidation of loop diuretic furosemide at gold electrode and its analytical applications. Intl J Electrochem Sc 4:104–121, http://www.electrochemsci.org/papers/vol4/4010104.pdf
Goss CA, Brumfield JC, Irene EA, Murray RW (1993) Imaging the incipient electrochemical oxidation of highly oriented pyrolytic graphite. Anal Chem 65:1378–1389. https://doi.org/10.1021/ac00058a014
Wood EL, Tatke A, Viehmann A, Ashtiani M, Friedman RL, Kopcha M, Fisher AC (2022) Dosage unit uniformity and dissolution testing of extended-release pharmaceutical products marketed in the US. Int J Pharm 625:122119. https://doi.org/10.1016/j.ijpharm.2022.122119
Funding
The authors are grateful to FONDECYT (Grant 1160167).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulppi, M., Irrazabal, J.P., Azócar, M.I. et al. The combination of extraction and chronocoulometry of a solid precipitate for teaching a novel approach of electroanalysis. J Solid State Electrochem 28, 677–682 (2024). https://doi.org/10.1007/s10008-023-05764-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05764-8