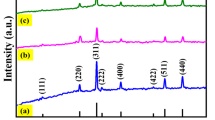
Abstract
Electrochemical detection of cadmium cations in aqueous solutions using a bentonite-modified carbon paste as working electrode was investigated. For this purpose, cyclic voltammetry, square wave voltammetry, and electrochemical impedance spectroscopy techniques were used. The findings demonstrated that the electrode composed of 20 mass% bentonite manifested high detection sensitivity, and hydrochloric acid was the best suitable supporting electrolyte. Under these operating conditions, the electron transfer process was quasi-reversible. Also, it was found that the increase of pH resulted in the decrease of the anodic current density, likely because of partial immobilization of the cadmium-derivative species (Cd2+, Cd(OH)+) by negatively charged clay particles. Furthermore, a linear relationship was observed between the initial concentration of Cd2+ (up to 5 × 10−5 M) and the anodic current density. The latter signal markedly declined as the Cd2+ ion concentration approached the cation exchange capacity of the electrode (about 6 × 10−6 M). The use of high potential scan rate (> 50 mV/s) led to the cathodic peak vanishing presumably because of the decline in the electron transfer between Cd2+ ions and the electrode. The used electrode was regenerated by using salt solution and successfully reused. Moreover, the co-presence of cadmium and lead ions did not alter its electrochemical performance. The electrode-solution interface was equivalent to an electrical circuit comprising a constant phase element in parallel with a resistance and a Warburg diffusion regime.














Similar content being viewed by others
References
López JE, Arroyave C, Aristizábal A, Almeida B, Builes S, Chavez E (2022) Reducing cadmium bioaccumulation in Theobroma cacao using biochar: basis for scaling-up to field. Heliyon 8(6):e09790
Zhang Y, Wu Y, Song B, Zhou L, Wang F, Pang R (2022) Spatial distribution and main controlling factor of cadmium accumulation in agricultural soils in Guizhou. China J Hazard Mater 424:127308
Wang Y, Xing W, Liang X, Xu Y, Wang Y, Huang Q, Li L (2022) Effects of exogenous additives on wheat Cd accumulation, soil Cd availability and physicochemical properties in Cd-contaminated agricultural soils: a meta-analysis. Sci Total Environ 808:152090
Agyeman PC, Borůvka L, Kebonye NM, Khosravi V, John K, Drabek O, Tejnecky V (2023) Prediction of the concentration of cadmium in agricultural soil in the Czech Republic using legacy data, preferential sampling, Sentinel-2, Landsat-8, and ensemble models. J Environ Manage 330:117194
Souza-Arroyo V, Fabián JJ, Bucio-Ortiz L, Miranda-Labra RU, Gomez-Quiroz LE, Gutiérrez-Ruiz MC (2022) The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 480:153339
Li Y, Rahman SU, Qiu Z, Shahzad SM, Nawaz MF, Huang J, Naveed S, Li L, Wang X, Cheng H (2023) Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: a review. Environ Pollut 325:121433
Naksen P, Boonruang S, Yuenyong N, Lee HL, Ramachandran P, Anutrasakda W, Amatatongchai M, Pencharee S, Jarujamrus P (2022) Sensitive detection of trace level Cd (II) triggered by chelation enhanced fluorescence (CHEF)“turn on”: nitrogendoped graphene quantum dots (N-GQDs) as fluorometric paper-based sensor. Talanta 242:123305
Blaise N, Gomdje Valéry H, Maallah R, Oubaouz M, Tigana Djonse Justin B, Andrew Ofudje E, Chtaini A (2022) Simultaneous electrochemical detection of Pb and Cd by carbon paste electrodes modified by activated clay. J Anal Methods Chem 2022
Thatikayala D, Noori MT, Min B (2023) Zeolite-modified electrodes for electrochemical sensing of heavy metal ions–progress and future directions. Mater Today Chem 29:101412
Selmi A, Khiari R, Snoussi A, Bouzouita N (2021) Analysis of minerals and heavy metals using ICP-OES and FTIR techniques in two red seaweeds (Gymnogongrus griffithsiae and Asparagopsistaxiformis) from Tunisia. Biol Trace Elem Res 199:2342–2350
Lemos VA, de Carvalho AL (2010) Determination of cadmium and lead in human biological samples by spectrometric techniques: a review. Environ Monit Assess 171:255–265
He Q, Wang B, Liang J, Liu J, Liang B, Li G, Long Y, Zhang G, Liu H (2023) Research on the construction of portable electrochemical sensors for environmental compounds quality monitoring. Mater Today Adv 17:100340
Hanrahan G, Patil DG, Wang J (2004) Electrochemical sensors for environmental monitoring: design, development and applications. J Environ Monit 6(8):657–664
Afsharara H, Asadian E, Mostafiz B, Banan K, Bigdeli SA, Hatamabadi D, Keshavarz A, Hussain CM, Keçili R, Ghorbani-Bidkorpeh F (2023) Molecularly imprinted polymer-modified carbon paste electrodes (MIP-CPE): a review on sensitive electrochemical sensors for pharmaceutical determinations. TrAC Trends Anal Chem 160
Mostafiz B, Bigdeli SA, Banan K, Afsharara H, Hatamabadi D, Mousavi P, Hussain CM, Keçili R, Ghorbani-Bidkorbeh F (2021) Molecularly imprinted polymer-carbon paste electrode (MIP-CPE)-based sensors for the sensitive detection of organic and inorganic environmental pollutants: a review. Trends Environ Anal Chem 32:e00144
Jayaprakash GK, Swamy BK, Rajendrachari S, Sharma SC, Flores-Moreno R (2021) Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications. J Mol Liq 334:116348
Shetti NP, Nayak DS, Reddy KR, Aminabhvi TM (2019) Graphene–clay-based hybrid nanostructures for electrochemical sensors and biosensors. In Graphene-based electrochemical sensors for biomolecules 235–274
Baranwal J, Barse B, Gatto G, Broncova G, Kumar A (2022) Electrochemical sensors and their applications: a review. Chemosensors 10(9):363
GadelHak Y, Hafez SH, Mohamed HF, Abdel-Hady EE, Mahmoud R (2023) Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: a review. Microchem J 193:109043
Muslim WA, Al-Nasri SK, Albayati TM (2023) Evaluation of bentonite, attapulgite, and kaolinite as eco-friendly adsorbents in the treatment of real radioactive wastewater containing Cs-137. Prog Nucl Energy 162:104730
Yıldız C, Bayraktepe DE, Yazan Z, Önal M (2022) Bismuth nanoparticles decorated on Na-montmorillonite-multiwall carbon nanotube for simultaneous determination of heavy metal ions-electrochemical methods. J Electroanal Chem 910:116205
Souza LP, Lima AR, Martins TA, Vicentini FC, Marcolino-Junior LH, Bergamini MF (2023) Electrochemical determination of vitamin B12 (cyanocobalamin) using mercury nanodroplets supported at montmorillonite on a carbon paste electrode (CPE) Anal Lett 1–16
Caglar B, GunerEK KK, Özdokur KV, Cubuk O, Coldur F, CaglarS TopcuC, Tabak A (2018) Fe3O4 nanoparticles decorated smectite nanocomposite: characterization, photocatalytic and electrocatalytic activities. Solid State Sci 83:122–136
Tsotsou GE, Mazarakis AP (2023) Prospects and limitations of a clay-enabled pre-concentration method for spectrophotometric quantification. Appl Clay Sci 233:106829
Jaber L, Elgamouz A, Kawde AN (2022) An insight to the filtration mechanism of Pb (II) at the surface of a clay ceramic membrane through its preconcentration at the surface of a graphite/clay composite working electrode. Arab J Chem 15(12):104303
Jlassi K, Al Ejji M, Ahmed AK, Mutahir H, Sliem MH, Abdullah AM, Chehimi MM, Krupa I (2023) A carbon dot-based clay nanocomposite for efficient heavy metal removal. Nanoscale Adv 5:4224–4232
Beltagi AM, Ismail IM, Ghoneim MM (2013) Square-wave adsorptive cathodic stripping voltammeteric determination of manganese (II) using a carbon paste electrode modified with montmorillonite clay. Am J Anal Chem 4(4):30455
Guenang LS, Dongmo LM, Jiokeng SL, Kamdem AT, Doungmo G, Tonlé IK, Bassetto VC, Jović M, Lesch A, Girault H (2020) Montmorillonite clay-modified disposable ink-jet-printed graphene electrode as a sensitive voltammetric sensor for the determination of cadmium (II) and lead (II). SN Appl Sci 2:1–13
Aran D, Maul A, Masfaraud JF (2008) A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. CR Geosci 340(12):865–871
Ilkhomidinovich MI (2019) Study of the sorption and textural properties of bentonite and kaolin. Austrian J Tech Nat Sci 11–12:33–37
Matusik J, Koteja-KuneckaA MP, Kunecka A (2022) Styrene removal by surfactant-modified smectite group minerals: efficiency and factors affecting adsorption/desorption. Chem Eng J 428:130848
Lu HL, Li KW, Nkoh JN, He X, Hong ZN, Xu RK (2022) Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 301:134674
Da Silva OB, Machado SA (2012) Evaluation of the detection and quantification limits in electroanalysis using two popular methods: application in the case study of paraquat determination. Anal Methods 4(8):2348–2354
Manisha H, Sonia J, Shashikiran S, Yuvarajan S, Rekha PD, Prasad KS (2022) Computer numerical control-printed paper electrodes for electrochemical detection of Pseudomonas aeruginosa virulence factor pyocyanin. Electrochem Commun 137:107259
Okpara EC, Fayemi OE, Sherif ESM, Ganesh PS, Swamy BK, Ebenso EE (2022) Electrochemical evaluation of Cd2+ and Hg2+ ions in water using ZnO/Cu2ONPs/PANI modified SPCE electrode. Sens Bio-Sens Res 35:100476
Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL (2018) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95(2):197–206
Coutinho I, Fortunato E (2018) A simple procedure to fabricate paper biosensor and its applicability—NADH/NAD+ redox system. J Pharm Pharmacol 6:175–187
Xinxin MO, Siebecker MG, Wenxian GOU, Ling LI, Wei LI (2021) A review of cadmium sorption mechanisms on soil mineral surfaces revealed from synchrotron based X-ray absorption fine structure spectroscopy: implications for soil remediation. Pedosphere 31(1):11–27
Arun Kumar NS, Ashoka S, Malingappa P (2018) Nano zinc ferrite modified electrode as a novel electrochemical sensing platform in simultaneous measurement of trace level lead and cadmium. J Environ Chem Eng 6(6):6939–6946
Hwang JH, Wang X, Pathak P, Rex MM, Cho HJ, Lee WH (2019) Enhanced electrochemical detection of multiheavy metal ions using a biopolymer-coated planar carbon electrode. IEEE Trans Instrum Meas 68(7):2387–2393
Lalmalsawmi J, Tiwari D, Lee SM (2020) Low cost, highly sensitive and selective electrochemical detection of arsenic (III) using silane grafted based nanocomposites. Environ Eng Res 25(4):579–587
Aghris S, Matrouf M, Ettadili FE, Laghrib F, El Bouabi Y, Saqrane S, Farahi A, Bakasse M, Lahrich S, El Mhammedi MA (2021) Electrochemical analysis of flubendiamide in water and white rice using clay microparticles supported on pencil electrode. Microchem J 168:106486
Alves TS, Santos JS, Fiorucci AR, Arruda GJ (2019) A new simple electrochemical method for the determination of bisphenol a using bentonite as modifier. Mater Sci Eng, C 105:110048
Lalmalsawmi J, Tiwari D, Lee SM, Kim DJ (2021) Indigenously synthesized nanocomposite materials: use of nanocomposite as novel sensing platform for trace detection of Pb2+. J Electroanal Chem 897:115578
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mourak, A., Hajjaji, M., Idoulhi, R. et al. Cadmium sensing with bentonite-modified carbon paste electrode: electrochemical insights. J Solid State Electrochem (2023). https://doi.org/10.1007/s10008-023-05702-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-023-05702-8