Abstract
Alloy electroplating using deep eutectic solvents (DES), compared to aqueous electrolytes, has essential benefits in a wide potential range, the high solubility of metal salts (including chlorides and oxides), and an environmentally friendly alternative. This research aimed to compare the coatings obtained by electrolysis of the Ni–Co alloy conventionally from an aqueous solution against the electroplating obtained in 2:1 ethylene glycol-choline chloride. The electrochemical behavior was studied through potentiodynamic polarization kinetic analysis carried out complying with Abner’s rules for alloy deposits; hydrodynamic conditions were modified, keeping the temperature at 60 °C and the salt concentration in both baths constant. As a result, nickel electrolysis has been carried out successfully by taking advantage of the benefits of using DES in energy consumption with 80% efficiency compared to water as a solvent under the same conditions. The composition and morphology of Ni–Co alloy coatings were characterized by scanning electron microscopy (SEM/EDS), and corrosion resistance was investigated by potentiodynamic polarization and electrochemical impedance (EIS). Coatings were obtained for both electrolytic baths with a chemical composition within the range of the alloy but with a higher cobalt content in the deposits obtained in water; in contrast, the distribution of the Ni–Co alloy was more homogeneous with changes in morphology and crystallization in the deposits obtained from the DES bath. The coatings’ anti-corrosion performance showed that the Co content difference increases the corrosion resistance of the Ni–Co alloy obtained from aqueous electrolytes compared to deposits obtained from DES.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloy electroplating is the final stage of production of some components, the purpose of which is to modify the surface properties of a material and thus provide numerous essential qualities and art to be manufactured. Electrochemical alloys often exhibit properties and performance that are notably better concerning wear resistance, hardness, and corrosion stability than those of individual components, even compared to metallurgical alloys.
In the world of alloy tanks, there are some variables to consider allowing optimal conditions for them to occur with the best value for money. Some issues or parameters need to be considered and are fascinating to implement, for example, the concentration of the electrolyte bath, the current and voltage battery, additives, and stirring. In recent decades, the possibility of decorative and functional coatings using deep eutectic solvents (DES) as electrolytes have been extensively investigated because of their chemical properties that allow an excellent range of compounds that are not always soluble in water and electrochemical improvement on the transport and migration of ions as there are no reactions of electrolysis of water. Another advantage is the narrow potential window, involving the evolution of gases with the subsequent phenomena of hydrogen embrittlement and the formation of insoluble oxides or hydroxides on the surface of the electrode (passivation) that hinder the deposition of thick metal coatings. An additional advantage is the energy limitation to deposit metals with redox potentials lower than the detachment of hydrogen, such as aluminum and magnesium, which are impossible to obtain through the usual processes of an aqueous solution.
Deep eutectic solvents (DES) were introduced in 2003 by Abbott et al. [1], furthermore, referred to as a mixture of two or more components forming a eutectic characterized by a melting point much lower in comparison/contrast of each of its elements separately [2]. Typically, these solvents comprise a halide salt, choline chloride (ChCl), and a donor of hydrogen bonds such as urea, ethylene glycol, and malonic acid. This homogeneous liquid is formed due to ethylene glycol’s ability to form hydrogen bonds with chlorinated choline anions.
Nickel metal coatings have been successfully obtained by dissolving dehydrated salts of nickel chloride in both ChCl:urea and ChCl:ethylene glycol [3,4,5,6]: the morphology of the coatings obtained from the DES baths is entirely different compared to the aqueous Ni coated, due to the other thermodynamics and kinetics of the two processes. Recent research shows that adding various components to DES-based electrolytes can change the microstructure, morphology, and redox behavior of the Ni (II) ion. Gu et al. [7] performed electrodeposition at room temperature and 90 °C using a ChCl:ethylene glycol bath on a brass sheet. This work demonstrates that surface roughness increases with temperature due to the formation of nanosheets with a thickness of 10 to 20 nm and a grain size of approximately 10 to 50 nm. This phenomenon is related to a decrease in the viscosity of DES that causes an increase in the mobility of ionic species, thus, favoring the process of deposition and nucleation of Ni. The coating obtained also shows a low corrosion potential. Abbot et al. [8] have also studied the electrodeposition of Ni–Co from the DES bath of ChCl:ethylene glycol, obtaining a dark gray deposit by adding ethylenediamine and acetyl acetamide in this type of electrolyte induces the suppression of the deposition of sub potential Ni. Other research reports the use of additives such as nicotinic acid and ethylenediamine as additives that modify the morphology and microstructure of the coating, show the interaction of compounds forming a complex with nickel, and improve the adsorption of the electrode surface [9, 10].
One of the alloys with which it presents greater use at an industrial level is Ni–Co due to its high corrosion resistance and its low coefficient of friction and magnetic properties. These nickel-based alloys have been successfully investigated from DES electrolytes with mixtures of choline chloride and ethylene glycol or urea, finding modifications in the structures made by electrodeposition improve thickness in complex geometries [11, 12]. Besides the increasing industrial interest in composite materials, several studies have been conducted to develop electroplating processes from DES. Compact Ni multilayer carbon nanotubes (MWCNT) were deposited using nickel as a matrix on a copper substrate. A homogeneous dispersion of MWCNT in the electrolyte must ensure a highly efficient deposition process and be obtained by dispersion of MWCNT in the ChCl:urea DES before adding soluble nickel chloride salt. The morphology, crystallization, and roughness of the nickel layer have been affected by the presence of MWCNT.
Nickel electroplating processes in aqueous solutions are carried out at a higher temperature (40 to 90 °C), which results in a lower electrolyte resistance, lowering the voltage [13,14,15]. On the other hand, it has been reported that deep eutectic solvents based on choline chloride and ethylene glycol present thermal instability and more significant degradation at high temperatures (100 °C) [16, 17]. Therefore, a temperature of 60 °C was selected for both solvents to guarantee ion migration conditions and kinematic viscosity and to avoid the precipitation of boric acid and other components.
In the present investigation, an analysis of electrochemical parameters was carried out where under the same conditions of concentration of salts in the electrolyte, temperature, and hydrodynamic conditions, the deposit of the Ni–Co alloy in water and DES was guaranteed. Once identified and characterized, the electrodeposition phenomenon of the alloy deposits was obtained by resistance to corrosion, and the resulting composition and morphology were discontinued.
Experimental procedures
Electrochemical evaluation
Electrolytes were cobalt sulfate solution (CoSO4), nickel plating solution (Watts), and modified Watt solution (DOX), and two solvents were used for electrochemical evaluations: a deep eutectics solvent (DES) and water. The DES used was choline chloride (ChCl) (Aldrich analytical reagents, USA) and ethylene glycol in a ratio of 1:2; no water was added to the bath as the ethylene glycol used is not an anhydride chemical reagent, and recent studies refer to the effect of water on tank quality obtained by DES [18,19,20]. The composition of nickel salts, cobalt salts, and boric acid of the electrolyte was adjusted for the reservoir baths, as shown in Table 1.
A typical three-electrode cell was used: a silver-silver chloride reference electrode (Cole Palmer, USA), a graphite rod counter electrode, and an AISI 304 stainless steel Teflon-insulated rotating disc with an area of 1 cm2 as the working electrode. All evaluations were carried out under different hydrodynamic conditions at 0, 25, and 50 rpm with a rotating disc electrode (ECR) (Pine Research, USA), and the temperature was kept constant at 60 °C with a heating grid (Thermo Fisher Scientific, USA). Electrochemical measurements were conducted on a potentiostat/galvanostat Gil AC (ACM Instruments, UK). The sequence was as follows: The open-circuit potential (OCP) was monitored for 900 s to reach a steady state. Next, a potentiodynamic polarization (PP) with a cathodic overpotential of – 1 V OCP with a sweep rate of 100 mV/min was applied. The experiments were conducted in triplicate. Kinetic analysis and electrochemical parameters were performed based on the results to produce a Ni–Co alloy. From the deposition current density in DOX solution, deposits were made in a Haring-Hull-type cell with AISI 1018 steel electrodes as cathode and high-purity nickel–cobalt plate as an anode, with surface preparation based on ASTM B254-92 [21] separation of the electrodes of 5 cm.
Ni–Co alloy coatings obtained from H2O and DES comparative analysis
After obtaining the alloy deposits, the parts were coated and cleaned with acetone in an ultrasonic vat for 15 min to be correctly analyzed by the SEM (JEOL-JCM-6000PLUS, USA). The coatings were characterized to identify the morphology of the deposit in both media, and the EDS chemical microanalysis and mapping of nickel and cobalt were present to analyze the distribution of chemical species.
The corrosion tests were conducted with a computer-controlled and software-supported Gill AC (ACM Instruments, UK) potentiostat–galvanostat. A typical three-electrode system consists of an alloy coating (1 cm2) as a working electrode, a graphite rod as a counter electrode, and a silver chloride (Ag/AgCl, 210 mV vs. NHE) as reference. The working electrode was rinsed and dried before measuring. The aqueous solution used for the corrosion resistance assessment was NaCl 3% wt. (Sigma Aldrich S5886 – 1 KG). The corrosion evaluation sequences are as follows: The open circuit potential (OCP) was monitored for 7200 s. Electrochemical impedance spectroscopy (EIS) has a CA disturbance voltage of 10 mVRMS vs. OCP and a frequency range of 104 to 10−2 Hz. After EIS, the linear polarization resistance (LPR) was carried out using ± 0.03 V overpotential vs. open-circuit potential at a 1 mV s−1 scan. Finally, potentiodynamic polarization (PP) measurements were carried out using ± 0.3 V over the previously determined open-circuit potential at a 1 m V s−1 scan.
Results and discussion
Electrochemical deposition of Ni–Co alloy coatings by H2O and DES
The potentiodynamic polarization for cobalt sulfate and Watt bath is shown in Fig. 1. The analysis is based on identifying the intersection in the polarization curves of cobalt and Watts; parameters are shown in Table 2.
For the analysis, Abner Brenner et al. [22, 23] indicated rules for generating an electrodeposition alloy where nickel and cobalt species’ mechanism and ionic kinetics migrate and shrink simultaneously. One of the limitations in developing this alloy is that although the redox potential of cobalt (− 0.28 V vs. NHE) is less active compared to the redox potential of nickel (− 0.257 V vs. NHE), cobalt is deposited with more ionic speed, and this phenomenon is known as a type of anomalous deposit. Some variables that may improve alloy deposition are the composition of ions in the solution, temperature, and electrolyte agitation.
In Fig. 1A, C, and E, changes in the open circuit potential are observed that change concerning the dynamic conditions evaluated in water; the average data are reported in Table 1; the equilibrium potential of cobalt shifts to anodic values concerning agitation, so it can be assumed that the thickness of the electrochemical double layer decreases. Moreover, the nickel potential does not affect the agitation of the solution; this is because the concentration of the species in the solution is supersaturated, and there is no effect on the concentration of species for persistent laminar conditions [24, 25], besides, in Fig. 1B, D, and E, the response of DES is observed under different dynamic conditions, where there is no effect on the equilibrium potentials for nickel and cobalt; this can be related to the temperature-viscosity dependence that is directly related to the increased ionic activity of the solvent [26], the activation energy and intermolecular forces in choline chloride-based solvents have a linear relationship with temperature, and the kinematic viscosity of the solution is also modified ion mobility and decrease in current output [27]. The behavior confirms the observations of other researchers that this class of DES may approximate the deposition potentials of many metals [28,29,30]. DES deposition initiation potentials are expected to vary from water because metal ions form different complexes. While Co(II) ions in an aqueous solution are expected to exist as hexahydrate complexes, they are likely to exist as anionic chlorine complexes in this DES, changing the electrochemical behavior of Co(II); this effect has also been reported in metallic chlorine compounds for Cu in choline chloride-based solvents [28]. The growth rate of nickel is observed to increase proportionally with the PP current from its depositional potential until the PP plateau is reached. At the same time, Co’s growth initially follows this expected behavior. However, significant overpotentials lead to a decrease in the film’s growth rate through a diffusive control mechanism.
In addition, a higher current density than ionic kinetics shows an intersection of bath Watts and cobalt sulfate due to the equalization of reduced velocities at the metal interface, which ensures that both ions are deposited at the same rate. When comparing those of the Watts solution and the cobalt solution in the DES solution (Fig. 1D), it was found that the increase in flux induces an increase in the recorded current density and a slight depolarization of the cathodic process compared to the PP of water under the same conditions; this is due to the convective effect—leading to the incorporation of the particles into the increasing deposit. The electrochemical kinetics of cobalt for both solvents is rapidly shifting from activation control (charge transfer) to mixed control (charge and mass transport) rather than ion transfer. It is rapidly limited by a diffusion phenomenon derived from the reduction of cobalt at the metal interface.
On the other hand, when the dynamic conditions of the solvents at 25 rpm are modified when analyzing the C and D plots, there is an intersection between the Watts and the cobalt bath, which promotes ionic mobility, and the effect of the double layer is reduced by presenting a depolarization and modification of the diffusive layer giving as a response modification mixed control. Furthermore, when an intersection is found in the polarization curves of the nickel and cobalt baths, it is assumed that at this current density the speed of the reduction kinetics is the same, thus ensuring that both ions are co-deposited simultaneously.
The potential required for the composition of nickel and cobalt ion at 25 rpm is shown at the intersection of − 0.421 0.025 V vs. Ag/AgCl and a current exchange density of 57.61 ± 4.105 μA cm − 2 in water, and an intersection of − 0.527 ± 0.036 V vs. Ag/AgCl and an exchange current density of 10.539 ± 7.247 μA cm − 2 in DES; the ions in aqueous solution for the alloying systems comply with the convergence of the deposition potentials for both components. Theoretically, the energy will result in the formation of the alloy, that is, purely thermodynamic factors when the cobalt ion cathode solution layer is depleted, generating polarization. OCP of nickel in a solution of − 0.122 ± 0.082 V vs. Ag/AgCl and of cobalt in a solution of − 0.328 ± 0.028 V vs. Ag/AgCl for DES, no intersection is observed at 0 rpm; however, in these dynamic conditions, the B system does not appear, which prevents that in these conditions the ions can codeposit at the same speed under a mechanism controlled by charge transfer. In addition, the electrochemical kinetics of cobalt for both solvents rapidly changes from an activation control (charge transfer) to a mixed control (charge and mass transport) rather than the ion transfer phenomenon. It is rapidly limited by a diffusion phenomenon derived from the reduction of cobalt at the metal interface.
Based on the findings, the ion deposition mechanism is similar for water and DES; the electroactive particles are transported by a potential difference to the electrode surface and then desorbed and reduced. Consequently, the maximum content in the coating is reached in the vicinity of the intersection potential of both polarizations where the flow of ions and migration are at the same speed. Therefore, exploiting one of the above approaches or a combination of results in the formation of an alloy at a particular potential E that can be expressed through the thermodynamic and kinetic parameters of both components (Eq. 1),
where aNi and aCo are the actual activities of the electroactive ions near the electrode, E0 (Ni) and E0 (Co) are the standard potentials of the corresponding redox electrodes, and ηNi and ηCo are the total overpotentials of the two reduction processes. Thus, the primary condition for the joint discharge of ions is generally written. This relationship should be interpreted as a guide to actual calculations since the value of the potential deposition changes concerning the mixing of the compounds in the electrolytic bath, the roughness of the cathode surface, and the formation of complexes [29].
Therefore, each alloy deposition system requires an individual analysis of each component’s discharge kinetics and the phenomena associated with forced convection that are not included in the discharge of the ions. A clear example of this effect on ion transport phenomena was presented by increasing agitation, which does not enhance this effect. At 50 rpm condition, there is no intersection in the polarization curves, the effect of the increase in agitation is detrimental, facilitating the mobility of cobalt ions by presenting a higher current density compared to nickel ions, which decreases ionic mobility. And the capability to be reduced and deposited decreases. In addition, mass transport increases current flow by a considerable decrease in the thickness of the diffusive double layer, resulting in an accelerated reduction rate for cobalt ions that limits the process of an alloy by diffusion. As a result, it is concluded that the ideal depositing conditions for both solvents are 25 rpm.
Figure 2 shows the potentiodynamic polarization curves in the DOX bath for aqueous electrolytes and DES at 25 rpm. The potentiodynamic polarization curves are analyzed in the limiting current, with the solvent with the modified Watt solution (DOX) as a variable.
Potentiodynamic polarization
It is observed that the equilibrium potential for the DOX bath for both solvent systems is directly related to the amount of nickel in a solution that was reported in Table 2; the effect is presented in the current density where a decrease in the presence of CoSO4 is observed, which provides additional support that the presence of Co (II) in solution inhibits nickel deposition. Hence, the concentration of Co (II) in the design of baths for electroplating is less than 10% in the DOX solution either in aqueous electrolytes or DES; the same tendency of Ni (II) and the effect of cobalt to inhibit the codeposit of both is revealed. This effect of Co (II) concentration on Ni (II) deposition could be due to any of the three factors mentioned above (i.e., differences in surface coverage by adsorbed intermediates, Co(II) reduction catalysis, and differences in charge transfer rates). The response to the overpotential applied to both solvents shows a notable decrease in current density for DES compared to water. For the DOX bath in DES solution, the current density increases rapidly about the overpotential and presents an inflection denoted as a point I to a potential envelope of − 0.3 V vs. Ag/AgCl, giving rise to a mechanism controlled by charge transport where nickel and cobalt begin to reduce and co-deposit for the deep eutectic solvent this process requires a lower amount of energy compared to the process that develops in water, in the curve is denoted as point II; therefore, in both areas shown, Ni (II) and Co (II) are reduced in the same range of overpotential for both electrolytes and the surface of the electrode.
As previous results show, water reduction tends to occur significantly only when other reduction reactions have reached limiting mass transfer conditions. Consequently, it is reasonable to expect that not much hydrogen will be reduced from the electrolysis reaction of water has occurred when an electrode potential of – 0.8 V is reached during exploration in the DOX bath for both solvents; for the DOX bath in DES solvent, the potential and current density decreases and presents a bending in the same potential already mentioned this peak is denoted as III, this may be due to the decomposition potential of choline chloride or attributed to a chelating effect of choline chloride with Ni (II) and Co (II)ions and its subsequent adsorption and reduction of the electrode surface DES [30,31,32,33], since the flow conditions keep the species from solution to the electrode surface constant and is probably not related to the fact that the mass transport of any of the electroactive species to relate it to a limiting current but for the DOX polarization curve in water showed an increase in current density, well above the limit current values for the reduction of H + , Co(II), and Ni(II). This transition is marked by another tipping point that reflects the onset of the water reduction we call region IV. [34].
The current efficiency of the deposits (%) measures the percentage of the total charge used for the reduction of Co (II) and Ni (II) in the deposit process. As a result, it reflects the contribution of hydrogen shedding (e.g., H + and water reduction) to the total current density generated in the system. To calculate this current efficiency obtained during the galvanostatic experiments, a relationship has been determined between the total mass (i.e., cobalt and nickel) deposited and the theoretical mass calculated using Faraday’s law on the assumption that there is no hydrogen evolution. The results for the individual baths included in this study are presented in Table 3.
These phenomena decrease the nickel deposition current and its nucleation, involving a significant decrease in the energy required to perform the alloy deposition compared to water as an electrolyte; the current was selected since the alloy electroplating mechanism is by a diffusion control; although no limit current is observed in the DES bath, the same calculation was obtained from the last recorded current density.
Perhaps the most notable characteristic is the general tendency in which the efficiency of the net current is lower for DOX baths in DES to lower potentials than DOX baths in water. At low overpotentials, the reduction of H is the first reaction before the Ni (II) and Co (II) reduction are fully activated. As the current increases, the potential becomes negative because of the beginning of the reduction of Ni (II) and Co (II). Once these reactions are restricted to mass transfer at about − 1 V, the efficiency decreases due to the initiation of water reduction.
It highlights current density changes in DES and the time required for alloy deposition, resulting in greater efficiency in reducing the ion transport effect of choline chloride compared to the electrolysis of water that releases two hydrogen protons for each exchange molecule; energy decreases because the electrolysis reaction of water consumes much more current.
Reactions (2)–(7) describe the global electrochemical reactions in both baths; reduction of the noble and active species (Ni2+ and Co2+) can be shown as.
In a water bath, the reaction can be shown as reactions (8)–(11):
The equations introduce the process of chemical decomposition of water during electrolysis, known as Hoffman removal. In a DES bath, reaction (12) shows the global reaction and reactions (13) and (14) show the reduction of ethylene glycol and choline chloride [35,36,37,38].
The electrochemical reaction promotes the formation of the electrochemical double layer, the discharge of diatomic ions on the metal surface, and the decrease of the energy necessary to reduce and deposit the Ni–Co alloy. Electrochemical reactions for choline chloride involve these composite protons carrying each ion at the metal interface by a chelating effect with nickel and cobalt [32, 39,40,41,42]. Moreover, the evolution of hydrogen produces a polarization of the concentration on the surface of the cathode, which prevents the migration of the charge transport through the double diffusion layer; it has also been reported that the predominant reaction occurring beyond metal deposition limiting the current plateau is the breakdown of choline to form trimethylamine gas [43].
In DES, the mechanism provides hydrogen by molecule and enhances the ionic mobility of nickel and cobalt. These parameters were deposited on the AISI 1018 carbon plate in 30 min. After deposition, they were cut and cleaned for corrosion testing and morphological and chemical analyses.
Morphologies and chemical composition
The morphology of the coatings produced was characterized by scanning electron microscopy (SEM) and composition by Rx emission. The SEM images are shown in Fig. 3.
Figure 3 shows images obtained from SEM with different magnifications (× 1000 and × 5000) to exhibit the changes in the deposit morphology; both coatings present cubic layers as an intermediate structure between blocks and lamellas [44]. Nonetheless, the changes in agglomeration and distribution are illustrated by comparing images obtained from water and DES baths. Although the main determining factor in the variation of the growth form of a given system may be the potential, this may mean the changes in morphology, in addition to the activation heat for charge transfer which is a function of position, giving as a result that the potential energy of the final state depends on the intermolecular potential of the solvents used, modifying how it will be deposited and in what position the newly formed adion is found. Therefore, although the overpotential (essentially a macroscopic quantity) and current density were determined from kinetic analysis to achieve the same deposition, the position and growth strongly depend on the electrochemical nature of the solvent, thus affecting morphological characteristics and distribution [45, 46].
In addition, Fig. 3 shows the thicknesses of both coatings on the cross-section of the deposition; the mean value was 20 μm ± 1.8 for the two layers, corresponding to the mean of several measurements carried out over different zones covering the whole surface. Contrary to expectations, the resulting structure does not fully show some degree of crystallization because there is no “segregation” of the deposited elements.
Although there were differences in the distribution of the deposits obtained in A and B, the observed morphology shows some similarities. Due to the kinetic analysis previously performed, the alloy should be achieved in both cases. The presentation of similarities gives indisputable veracity that it has been completed. Chemical mapping and analysis were also conducted to confirm and validate the kinetic analysis. Chemical studies of the electrolytic deposition of DOX alloys show a homogeneous morphologic structure composed of nickel and cobalt in an alloy matrix. Even though the matrix is made up of a nickel–cobalt alloy, the nickel–cobalt ratio is determined by the overall composition of the layer.
Figure 4 shows the quantitative chemical microanalysis to determine the composition ratio of nickel and cobalt (a) in an aqueous solvent and (b) DES in the alloy tank and to chemically evaluate, if successful, the alloy formed.
In both instances, an alloy deposition is present. However, the presence of the chemical species differs by up to 20%, with the DES solvent having the lowest percentage of cobalt, although 5% of cobalt and in aqueous solvents up to 23%; therefore, in both DES and an aqueous solvent, the alloy deposit was achieved within acceptable parameters [47,48,49]. However, it is necessary to emphasize the difference between the concentrations of cobalt obtained since some measurements made directly on the coating’s surface indicate that the amount of cobalt on it is more significant than that determined in the cross-sections [2, 50]. The latter is a consequence of a phenomenon called “riding,” first mentioned by White and Foster [51]; this phenomenon occurs because the particles are separated from the electrode by a thin layer (due to hydration forces) in which they diffuse the electroactive ions, which are then reduced under the particles. As a result, the crystalline growth exceeds a certain critical thickness, and the already reduced ions begin to co-deposit when definitively anchored to the coating, resulting in the cobalt composition in the layer obtained in water presenting a better chemical composition than in the alloy obtained by DES [52, 53].
Consequently, it allows us to understand the morphological differences found since the variation in the composition of cobalt favors the change in the morphology of the deposit. To better identify what was observed in the analysis of the chemistry of the leg, a mapping was carried out to compare the distribution of nickel and cobalt in the two coatings.
Figure 5 shows the analysis obtained from mapping the coating to determine the distribution of cobalt and nickel in the alloy obtained from the two solvents.
The nickel–cobalt alloy, by electroplating, forms a solid solution of infinite substitution [49]; if the chemical composition of the cobalt is weak, the crystalline structure can be ensured to grow. However, the cobalt level is higher in the coating obtained in water as an electrolyte, indicating that the DES bath’s design can considerably increase the amount of cobalt in solution by not performing as an anomalous type occurs in a water bath. Therefore, agglomerations are shown in the distribution (red color), which leads to the assumption that the film’s crystallization growth mechanism presents different microcrystalline structures with hcp crystalline structures that allow a higher stoichiometric ratio of cobalt in the alloy [54,55,56].
The mapping composition analysis corresponds to the results obtained with the electrochemical kinetics and the deposit morphology shown above. Ni–Co electroplating in DES electrolytes does not follow the anomalous co-deposition process [49] observed in aqueous electrolytes. Furthermore, as observed for solvents, water, and DES, the alloy was deposited but with different cobalt concentration ratios; the literature of various investigations [48, 49, 57] indicates that the ranges of the alloy that presents the best anticorrosive and mechanical properties are between 4 and 25% by weight of cobalt [48, 49, 57, 58]. Corrosion resistance measurements were made to electrochemically characterize the differences between both coatings and distinguish whether the differences in the composition of the layers are reflected in their performance and behavior as corrosion protection.
Corrosion performance of Ni–Co coatings
Figure 6 shows the Nyquist and Bode diagrams for both coatings; electrochemical impedance exhibits the same anticorrosive electrochemical response; equivalent electrical circuits (ECC) were used to calculate the parameters of charge transfer resistance and double-layer capacitance. Additionally, a modified Randle electric circuit was used to define the values. The results are presented in Table 4.
The coating resistance shows values of 2357 Ω cm2 for the tank obtained in water and 1055 Ω cm2 for DES. The electrochemical response is related to the difference in the concentration of the coatings obtained and the differences in the morphology of the compact tank since the thickness of the layer does not change. The coating capacitance for the two deposits has a characteristic value of a metal film with media of 9.43 × 10−7 ± 0.24 F for coating obtained by water solvent and 8.13 × 10−6 ± 0.89 F for DES, which are values associated with metal protective films. The resistance to charge transfer does not show significant differences; the phenomenon controlled by ion transfer at the interface shows the same resistive response for both tanks evaluated.
However, the deposited alloy changes were exhibited in the double-layer electrochemical capacitance for each coating; deposits generated in aqueous solution present an on value, compared to DES deposits showing a capacitance value. Additionally, the effect of the cobalt concentration difference changes the electrical interface with a higher capacitive response. Such behavior was anticipated due to the morphology and composition of each deposited alloy. However, the electrochemical reaction is very similar, but the composite ratio of the alloy obtained in DES could be improved in future work.
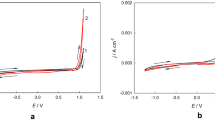
Potentiodynamic polarization (PP) was performed to assess system response, but in DC, the results are shown in Fig. 7, and Tafel extrapolation is shown in Table 5.
The potentiodynamic polarization of the coating obtained by water and DES shows similar electrochemical behavior; the corrosion potential changes with the increase of the Co content with a value of – 0.812 V vs. corrosion potential EAg/AgCl for coatings obtained for H2O and – 0.785 V in comparison to EAg/AgCl for DES. However, potential changes appear insignificant in response to changes in deposition composition and morphology reported by SEM. The charge-controlled mechanism of the anodic reaction has an anodic slope of − 0.229 V/DEC for deposits in H2O, showing a lower reaction rate than the deposits obtained in DES, which gives a higher cathodic reaction rate with a slope of − 0.124 V/DEC. Another change in the anodic polarization of the coating obtained in water is shown; a difference in the mechanism was observed when changing the slope in a potential of − 0.7 V vs. EAg/AgCl, associated with the pseudo-passivation of the coating, the effect of the increase of cobalt in the chemical composition. In addition, the corrosion current density is higher for the alloy obtained by DES with a value of 0.927 μA cm−2 compared to H2O with a value of 0.336 μA cm−2. Electrochemical analysis showed that the Co content affects the corrosion resistance of the resulting deposits. However, both coatings exhibit similar electrochemical behavior regardless of the method of entering the aqueous solution or DES.
The change in the evaluated electrochemical response of both coatings is assumed to be the changes in composition and morphology, as reported by Yanai T et al. [59]; they show the electrochemical response changes with the design, morphology, and current of deposits of this alloy obtained by DES. Li W. et al. [8] report the effect on the anticorrosive response of Ni–Co films concerning bath salts, resulting in the composition of the choline chloride solution and the sensitivity in crystalline growth with the potential change of crystalline state to an amorphous state; they also present the same electrochemical response to changes in the cobalt composition in the Ni–Co alloy having the best corrosion resistance with 4.89% Co.
Summarizing the analysis of this research, it demonstrated the possibility of obtaining coatings from deep eutectic solvents based on choline chloride and ethylene glycol presents a remarkable energetic advantage in comparison with water as traditional this coating is manufactured, which approximates each industrialization in the continuous operation of metallic coatings. We demonstrated the electroplating of N–Co alloys from a DES based on ChCl-EG and electrolytic bathed water containing NiSO4∙ 6H2O, NiCl2∙6H2O and CoSO4∙6H2O at 60 °C under the same agitation conditions (25 rpm). The comparative requirements are given in Table 6.
Comparative studies of deposition mechanism, microstructure, and electrochemical properties between Ni and Ni–Co alloy films were examined. According to other researchers, this class of DES can approximate the deposition potential of many metals. The priming potentials of DES deposits are expected to vary from those of water because metal ions form different complexes. While Co (II) ions in an aqueous solution are expected to exist as hexahydrate complexes, they are likely to exist as anionic chlorine complexes in this DES, changing the electrochemical behavior of Co (II). This effect was also reported in the Cu metal chlorine compounds in choline chloride-based solvents. It is found that the growth rate of nickel increases in proportion to the CV current from its depositional potential until the CV shelf is reached. At the same time, cobalt’s growth initially follows this expected behavior. However, significant overpotentials lead to a decrease in the film’s growth rate through a diffusive control mechanism. Evidence of anomalous behavior was shown during alloy coding for conditions in which H + reduction is not controlled by mass transport in DES baths. Therefore, the reduction of H + does not need to be limited by mass transfer for anomalous behavior to be observed, as has been proposed in the past. As a result, deposition is not controlled by a diffuse phenomenon comparable system with water as a solvent.
The current density of deposition decreased by 20%. The surface morphology and chemistry of these films depend on the activity of nickel and cobalt in the solvents. The surface morphology and chemistry of these films depend on the activity of nickel and cobalt in the solvents. Ni–Co electrodeposition in DES-based electrolytes does not follow the anomalous co-deposition process, which usually occurs in aqueous electrolytes. The Ni–Co alloys deposited in DES have a refined grain deposition morphology with a homogeneous distribution of the alloying elements compared to aqueous electrolytes that present a random segregation of cobalt. The Ni–Co alloys deposited in DES have a refined grain deposition morphology with a homogeneous distribution of the alloying elements compared to aqueous electrolytes that present a random segregation of cobalt. Measurements of the corrosion resistance of both coatings indicate that the more Co content the Ni–Co films have, the more negative corrosion potential and corrosion current the films exhibit.
Conclusions
The research aimed to optimize the operating conditions to generate a Ni–Co alloy from DES; it was possible to obtain dense coatings comparable to coatings obtained in baths with water as a solvent but with decreased deposit current density and time. Based on the investigation of the deposition mechanism, it may be postulated that the changes in the reduction and interference of the electrolysis of water and choline chloride have mechanisms of different transports and migrations of ions; it is concluded that the electrolysis of DES generated a cathodic depolarization and a co-deposition of cobalt and nickel with a decrease in exchange current increasing.
Evidence of anomalous performance was shown during alloy coding for conditions in which H+ reduction is not controlled by mass transport in DES baths. Therefore, the reduction of H+ should not be restricted by mass transfer for anomalous behavior to be observed; as has been proposed, the deposit is not controlled by a diffusive phenomenon as in systems with water as a solvent.
In contrast, the characterization of the deposits obtained by SEM determined that the alloys obtained by the two electrolytic media have a similar composition of nickel and cobalt in the alloy range. However, hydrogen concentration decreases at the metal electrolyte interface, and the distribution of elements in aqueous deposits was heterogeneous compared to DES deposits with better distribution. The different alloys’ distribution was related to the corrosion performance of the coating, the content of Co, the distribution, and the morphology of the alloy, modifying the corrosion resistance of the deposits obtained. Nevertheless, the two coatings have a similar electrochemical behavior regardless of the solvent obtained in the aqueous solution or DES.
References
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah VJ (2003) Chem Comm 70–71. https://doi.org/10.1039/B210714G
Zamani M, Amadeh A, Baghal SL (2016) J Transactions of Nonferrous Metals Society of China 26(2):484–491. https://doi.org/10.1016/S1003-6326(16)64136-5
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) J Chemical Society Reviews 41(21):7108–7146. https://doi.org/10.1039/C2CS35178A
Abbott AP, Harris RC, Ryder KS, D’Agostino C, Gladden LF (2011) Mantle MDJGC. J Green Chemistry 13(1):82–90. https://doi.org/10.1039/C0GC00395F
Figueiredo M, Gomes C, Costa R, Martins A, Pereira CM, Silva F (2009) J Electrochimica Acta 54(9):2630–2634. https://doi.org/10.1016/j.electacta.2008.10.074
Abbott A, El Ttaib K, Ryder KS, Smith E (2008) J Transactions of the IMF 86(4):234–240. https://doi.org/10.1179/174591908X327581
Gu C, Tu J (2011) J Langmuir 27(16):10132–10140. https://doi.org/10.1021/la200778a
Li W, Hao J, Mu S, Liu W (2020) J Appl Surf Sci 507:144889. https://doi.org/10.1016/j.apsusc.2019.144889
Yang H, Guo X, Birbilis N, Wu G, Ding W (2011) J Applied Surface Science 257(21):9094–9102. https://doi.org/10.1016/j.apsusc.2011.05.106
Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU (2006) J Chem Eng Data 51(4):1280–1282. https://doi.org/10.1021/je060038c
Winiarski J, Cieślikowska B, Tylus W, Kunicki P, Szczygieł B (2019) J Applied Surface Science 470:331–339. https://doi.org/10.1016/j.apsusc.2018.11.155
Li W, Hao J, Liu W, Mu S (2021) J Alloys Comp 853:157158. https://doi.org/10.1016/j.jallcom.2020.157158
Davis JR (2000) ASM International
Dennis JK, Such TE (2013) Elsevier Science
Panda H (2016) Asia Pacific Business Press Inc
Marchel M, Cieśliński H, Boczkaj G (2022) J Industrial Engineering Chemistry Research 61(30):11288–11300. https://doi.org/10.1021/acs.iecr.2c01898
Peeters N, Janssens K, de Vos D, Binnemans K, Riaño S (2022) J Green Chemistry 24(17):6685–6695. https://doi.org/10.1039/D2GC02075K
Lukaczynska M, Ceglia A, Van Den Bergh K, De Strycker J, Terryn H, Ustarroz J (2019) Electrochim Acta 319:690–704. https://doi.org/10.1016/j.electacta.2019.06.161
Cherigui EAM, Sentosun K, Mamme MH, Lukaczynska M, Terryn H, Bals S, Ustarroz J (2018) J Phys Chem C 122(40):23129–23142. https://doi.org/10.1021/acs.jpcc.8b05344
Mernissi Cherigui EA, Sentosun K, Bouckenooge P, Vanrompay H, Bals S, Terryn H, Ustarroz J (2017) The Journal of Physical Chemistry C 121(17):9337–9347. https://doi.org/10.1021/acs.jpcc.7b01104
ASTM-B254–92 (2020) ASTM International
Brenner A (1963) Academic Press Elsevier
Brenner A, Riddell GE (1998) Plat Surf Finish 85(8):54–56
Abbott AP, Capper G, Davies DL, Rasheed R (2004) Inorg Chem 43(11):3447–3452. https://doi.org/10.1021/ic049931s
Florindo C, Oliveira FS, Rebelo LPN, Fernandes AM, Marrucho IM (2014) Acs Sustainable Chemistry & Engineering 2(10):2416–2425. https://doi.org/10.1021/sc500439w
Moradi H, Farzi N (2021) J Mol Liq 339:116669. https://doi.org/10.1016/j.molliq.2021.116669
Abbott AP, Al-Barzinjy AA, Abbott PD, Frisch G, Harris RC, Hartley J, Ryder KS (2014) Phys Chem Chem Phys 16(19):9047–9055. https://doi.org/10.1039/C4CP00057A
Lo N-C, Chung P-C, Chuang W-J, Hsu SC, Sun I-W, Chen P-Y (2015) J Electrochem Soc 163(2):D9. https://doi.org/10.1149/2.0221602jes
Gamburg YD, Zangari G (2011) Springer Science & Business Media
Costa JGR, Costa JM, Almeida Neto AFd (2022) Metals 12(12):2095. https://doi.org/10.3390/met12122095
Protsenko VS, Kityk AA, Shaiderov DA, Danilov FI (2015) J Mol Liq 212:716–722. https://doi.org/10.1016/j.molliq.2015.10.028
Alesary HF, Ismail HK, Shiltagh NM, Alattar RA, Ahmed LM, Watkins MJ, Ryder KS (2020) J Electroanal Chem 874:114517. https://doi.org/10.1016/j.jelechem.2020.114517
Vieira L, Whitehead AH, Gollas B (2013) ECS Trans 50(52):83–94. https://doi.org/10.1149/05052.0083ecst
Alhaji AI (2012) University of Leicester
Abbott AP, Frisch G, Ryder KS (2008) Annual Reports Section “A” (Inorganic Chemistry) 104:21–45. https://doi.org/10.1039/B716593P
Cocalia VA, Gutowski KE, Rogers RD (2006) Coord Chem Rev 250(7–8):755–764. https://doi.org/10.1016/j.ccr.2005.09.019
Shirazinia SR, Semnani A, Nekoeinia M, Shirani M, Akbari A (2020) J Mol Liq 301:112364. https://doi.org/10.1016/j.molliq.2019.112364
Al-Murshedi AYM, Al-Yasari A, Alesary HF, Ismail HK (2020) Chem Pap 74(2):699–709. https://doi.org/10.1007/s11696-019-01025-z
Juma JA, Karim WO, Aziz SA, Omer KM (2021) Electrochemistry 89(6):602–605. https://doi.org/10.5796/electrochemistry.21-00087
Hayler HJ, Perkin S (2022) Chem Commun (Camb) 58(91):12728–12731. https://doi.org/10.1039/d2cc04008e
Smith E (2013) Transactions of the IMF 91(5):241–248. https://doi.org/10.1179/0020296713Z.000000000120
Liu K, Huang S, Jin Y, Ma L, Wang WX, Lam JC (2022) J Hazard Mater 433:128702. https://doi.org/10.1016/j.jhazmat.2022.128702
Mares Badea M, Cojocaru A, Anicai L (2014) UPB Sci Bull Ser B 76(3):21–32
Suzuki T (1972) J Journal of Crystal Growth 16(1):80–82. https://doi.org/10.1016/0022-0248(72)90092-9
Fashu S, Gu C, Wang X, Tu J (2014) J Surface Coatings Technology 242:34–41. https://doi.org/10.1016/j.surfcoat.2014.01.014
Fashu S, Gu C, Zhang J, Zheng H, Wang X, Tu J (2015) J Journal of Materials Engineering Performance 24:434–444. https://doi.org/10.1016/j.surfcoat.2014.01.014
Villalobos JC, Del-Pozo A, Campillo B, Mayen J, Serna S (2018) Metals 8(5):351. https://doi.org/10.3390/met8050351
Sankara Narayanan TSN (2005) Rev Adv Mater Sci 9:130–177. http://www.ipme.ru/e-journals/RAMS/
Bekish YN, Grabchikov S (2013) Tsybul’skaya LS, Kukareko VA, Perevoznikov SS. Prot Met Phys Chem Surf 49(3):319–324. https://doi.org/10.1134/S2070205113030040
Bai A, Hu C-C (2002) J Electrochimica acta 47(21):3447–3456. https://doi.org/10.1016/S0013-4686(02)00281-5
White C, Foster J (1981) J Transactions of the IMF 59(1):8–12. https://doi.org/10.1080/00202967.1981.11870553
Schiavi PG, Altimari P, Zanoni R, Pagnanelli F (2016) J Electrochimica Acta 220:405–416. https://doi.org/10.1016/j.electacta.2016.10.117
Bakhit B, Akbari A, Nasirpouri F, Hosseini MG (2014) J Applied Surface Science 307:351–359. https://doi.org/10.1016/j.apsusc.2014.04.037
Bastos A, Zaefferer S, Raabe D, Schuh C (2006) J Acta materialia 54(9):2451–2462. https://doi.org/10.1016/j.actamat.2006.01.033
Golodnitsky D, Gudin N, Volyanuk G (2000) J Electrochem Soc 147(11):4156. https://doi.org/10.1149/1.1394034
Radadi RMA, Ibrahim MAM (2021) J Korean Journal of Chemical Engineering 38:152–162. https://doi.org/10.1007/s11814-020-0661-8
Karimzadeh A, Aliofkhazraei M, Walsh FC (2019) Surface Coatings Technology 372:463–498. https://doi.org/10.1016/j.surfcoat.2019.04.079
Gamboa S, Gonzalez-Rodriguez J, Valenzuela E, Campillo B, Sebastian P, Reyes-Rojas A (2006) Electrochim Acta 51(19):4045–4051. https://doi.org/10.1016/j.electacta.2005.11.021
Yanai T, Akiyoshi T, Yamaguchi T, Takashima K, Morimura T, Nakano M, Fukunaga H (2018) AIP Adv 8(5):056106. https://doi.org/10.1063/1.5007189
Acknowledgements
The authors gratefully acknowledge the technical support from Guillermina González Mancera.
Funding
The present research was funded by UNAM-PAPIIT 5000–9091 of Dr. Francisco Javier Rodríguez Gómez and UNAM-PAPIIT 5000–9224 of Dra. Paola Roncagliolo Barrera.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barrera, P.R., Rodríguez, C.E. & Gomez, F.J.R. Comparative assessment of Ni–Co coatings obtained from a deep eutectic solvent, choline chloride-ethylene glycol, and water by electroplating. J Solid State Electrochem 27, 3075–3089 (2023). https://doi.org/10.1007/s10008-023-05591-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05591-x