Abstract
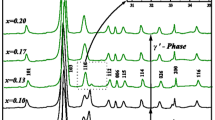
We report the effect of indium doping on thermal stability and ionic conductivity of beta alumina NaInxAl11-xO17 solid electrolyte which is synthesized by sol–gel auto combustion method on varying dopant concentration as x = 0, 0.1, 0.2, 0.3, and 0.4. X-ray diffraction (XRD) pattern and microstructure are investigated on the doped samples calcined at 1100 °C for 5 h. XRD confirms the change in unit cell volume on increasing the dopant concentration. Field emission scanning microscope (FESEM) reveals the conversion of cylindrical morphology to small spherical particles at dopant concentration x ≥ 0.5. Thermal stability is found to improve drastically over a broad temperature range even at small dopant concentrations in β-alumina as found from thermo-gravimetric analysis (TGA). Electrochemical impedance spectroscopy (EIS) shows a considerable reduction in frequency-dependent dielectric permittivity for doped beta alumina. At frequency 1 kHz, the permittivity of around ~ 105 in as-prepared shows non-monotonous dependence and decreases to 103 for x ≥ 0.1. This steep variation is mainly attributed to the change in morphology caused by steric effect and formation of random clusters which reduces the net polarization.
Graphical Abstract










Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Kim T, Song W, Son DY, Ono LK, Qi Y (2019) Lithium-ion batteries: outlook on present, future, and hybridized technologies. J Mater Chem A 7(7):2942–2964. https://doi.org/10.1039/C8TA10513H
Wang Y, Song S, Xu C, Hu N, Molenda J, Lu L (2019) Nano Materials Science Development of solid-state electrolytes for sodium-ion battery – a short review. Nano Materials Science 1(March):91–100. https://doi.org/10.1016/j.nanoms.2019.02.007
Li J, Liu C, Miao C, Kou Z, Xiao W (2021) Enhanced ionic conductivity and electrochemical stability of Indium doping Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes for all-solid-state lithium-ion batteries. Ionics 7(0123456789). https://doi.org/10.1007/s11581-021-04310-8
Tel’Nova GB, Solntsev KA (2015) Structure and ionic conductivity of a beta-alumina-based solid electrolyte prepared from sodium polyaluminate nanopowders. Inorg Mater 51(3):257–266. https://doi.org/10.1134/S0020168515030176
Li H, Zhang T, Yang Z, Shi Y, Zhuang Q, Cui Y (2021) Electrochemical impedance spectroscopy study on using Li10GeP2S12 electrolyte for all-solid-state lithium batteries. Intern J Electrochem Sci 16(2):1–13. https://doi.org/10.20964/2021.02.33
Lu X, Xia G, Lemmon JP, Yang Z (2010) Advanced materials for sodium-beta alumina batteries: Status, challenges and perspectives. J Power Sources 195(9):2431–2442. https://doi.org/10.1016/j.jpowsour.2009.11.120
Liu Z, Chen J, Wang X, Wang Y, Wang D, Mao Z (2020) Synthesis and characterization of high ionic-conductive sodium beta-alumina solid electrolyte derived from boehmite. J Mater Sci: Mater Electron 31(20):17670–17678. https://doi.org/10.1007/s10854-020-04321-7
Mali A, Petric A (2012) Synthesis of sodium β’-alumina powder by sol-gel combustion. J Eur Ceram Soc 32(6):1229–1234. https://doi.org/10.1016/j.jeurceramsoc.2011.11.004
Agustina AI, Skadell K, Dirksen CL, Schulz M, Kusumocahyo SP (2019) Sol-gel method for synthesis of Li+-stabilized Na-β"-alumina for solid electrolytes in sodium-based batteries. AIP Confer Proc 2175(November). https://doi.org/10.1063/1.5134634
Wei X, Cao Y, Lu L, Yang H, Shen X (2011) Synthesis and characterization of titanium doped sodium beta″-alumina. J Alloy Compd 509(21):6222–6226. https://doi.org/10.1016/j.jallcom.2011.03.006
Kim HJ, Krishna TNV, Zeb K, Rajangam V, Muralee Gopi CVV, Sambasivam S, Obaidat IM (2020) A comprehensive review of li-ion battery materials and their recycling techniques. Electronics (Switzerland) (Vol. 9). https://doi.org/10.3390/electronics9071161
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5(3):5884–5901. https://doi.org/10.1039/c2ee02781j
Skundin AM, Kulova TL, Yaroslavtsev AB (2018) Sodium-ion batteries (a review). Russ J Electrochem 54(2):113–152. https://doi.org/10.1134/S1023193518020076
Stevens DA, Dahn JR (2000) High capacity anode materials for rechargeable sodium-ion batteries. J Electrochem Soc 147(4):1271. https://doi.org/10.1149/1.1393348
Dai H, Xu W, Hu Z, Chen Y, Wei X, Yang B, Wei W (2020) Effective approaches of improving the performance of chalcogenide solid electrolytes for all-solid-state sodium-ion batteries. Front Ener Res 8. https://doi.org/10.3389/fenrg.2020.00097
Wang LP, Yu L, Srinivasan M, Xu ZJ, Wang X (2015) Recent developments in electrode materials for sodium-ion batteries. J Mater Chem A 3(18):9353–9378. https://doi.org/10.1039/c4ta06467d
Chandra A, Chandra A, Dhundhel RS (2020) Electrolytes for sodium ion batteries: a short review. Indian J Pure Appl Phys 58(2):113–119
Sun XG, Zhang Z, Guan HY, Bridges CA, Fang Y, Hu YS, Dai S (2017) A sodium-aluminum hybrid battery. J Mater Chem A 5(14):6589–6596. https://doi.org/10.1039/C7TA00191F
Hwang JY, Myung ST, Sun YK (2017) Sodium-ion batteries: present and future. Chem Soc Rev 46(12):3529–3614. https://doi.org/10.1039/c6cs00776g
Li F, Wei Z, Manthiram A, Feng Y, Ma J, Mai L (2019) Sodium-based batteries: from critical materials to battery systems. J Mater Chem A 7(16):9406–9431. https://doi.org/10.1039/c8ta11999f
Arya A, Sharma AL (2018) Structural, electrical properties and dielectric relaxations in Na+-ion-conducting solid polymer electrolyte. J Phys: Condens Matter 30(16):1–57. https://doi.org/10.1088/1361-648X/aab466
Wu EA, Banerjee S, Tang H, Richardson PM, Doux JM, Qi J, Ong SP (2021) A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries. Nat Commun 12(1):1–11. https://doi.org/10.1038/s41467-021-21488-7
Tanibata N, Takimoto S, Nakano K, Takeda H, Nakayama M, Sumi H (2020) Metastable chloride solid electrolyte with high formability for rechargeable all-solid-state lithium metal batteries. ACS Materials Letters 2(8):880–886. https://doi.org/10.1021/acsmaterialslett.0c00127
Dong H, Li Y, Liang Y, Li G, Sun CJ, Ren Y, Yao Y (2016) A magnesium-sodium hybrid battery with high operating voltage. Chem Comm 52(53):8263–8266. https://doi.org/10.1039/c6cc03081e
Yu X, Manthiram A (2019) Sodium-sulfur batteries with a polymer-coated NASICON-type sodium-ion solid electrolyte. Matter 1(2):439–451. https://doi.org/10.1016/j.matt.2019.03.008
Zhang R, Tutusaus O, Mohtadi R, Ling C (2018) Magnesium-sodium hybrid battery with high voltage, capacity and cyclability. Front Chem 6:1–10. https://doi.org/10.3389/fchem.2018.00611
Fergus JW (2012) Ion transport in sodium ion conducting solid electrolytes. Solid State Ionics 227:102–112. https://doi.org/10.1016/j.ssi.2012.09.019
Che H, Chen S, Xie Y, Wang H, Amine K, Liao XZ, Ma ZF (2017) Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ Sci 10(5):1075–1101. https://doi.org/10.1039/c7ee00524e
Devi C, Gellanki J, Pettersson H, Kumar S (2021) High sodium ionic conductivity in PEO/PVP solid polymer electrolytes with InAs nanowire fillers. Sci Rep 11(1):1–8. https://doi.org/10.1038/s41598-021-99663-5
Uvarov NF (2008) Composite solid electrolytes: recent advances and design strategies. J Solid State Electrochem 15(2):367–389. https://doi.org/10.1007/s10008-008-0739-4
Middlemiss LA, Rennie AJR, Sayers R, West AR (2020) Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep 6:232–241. https://doi.org/10.1016/j.egyr.2020.03.029
Sharma B, Sharma R, Kour S, Sharma MD, Amin O, Maity AR, Mukherjee R (2021) Fractional exponents of electrical and thermal conductivity of vanadium intercalated layered 2H-NbS2 bulk crystal. Indian J Phys 4–8. https://doi.org/10.1007/s12648-021-02045-w
Wang T, Su D, Shanmukaraj D, Rojo T, Armand M, Wang G (2018) Electrode materials for sodium-ion batteries: considerations on crystal structures and sodium storage mechanisms. Electrochemical Energy Reviews (Vol. 1). Springer Singapore. https://doi.org/10.1007/s41918-018-0009-9
Aziz SB, Woo TJ, Kadir MFZ, Ahmed HM (2018) Journal of Science : Advanced Materials and Devices A conceptual review on polymer electrolytes and ion transport models. Journal of Science: Advanced Materials and Devices 3(1):1–17. https://doi.org/10.1016/j.jsamd.2018.01.002
Kappel A, André M et al (2017) A study of equivalent electrical circuit fitting to electrochemical impedance using a stochastic method. Applied Soft Computing Journal 50:183–193. https://doi.org/10.1016/j.asoc.2016.11.030
Fei H, Liu Y, Wei C, Zhang Y, Feng J, Chen C, Yu H (2020) Poly(propylene carbonate)-based polymer electrolyte with an organic cathode for stable all-solid-state sodium batteries. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica 36(5):1–6. https://doi.org/10.3866/PKU.WHXB201905015
Zeng G, Liu Y, Gu C, Zhang K, An Y, Wei C, Ni J (2020) A nonflammable fluorinated carbonate electrolyte for sodium-ion batteries. Wuli Huaxue Xuebao/Acta Physico - Chimica Sinica 36(5):3–7. https://doi.org/10.3866/PKU.WHXB201905006
Arya A, Sharma AL (2019) Electrolyte for energy storage/conversion (Li +, Na +, Mg 2+ ) devices based on PVC and their associated polymer: a comprehensive review. J Solid State Electrochem 23(4):997–1059. https://doi.org/10.1007/s10008-019-04203-x
Ahmed SA, Pareek T, Dwivedi S, Badole M, Kumar S (2020) LiSn2(PO4)3-based polymer-in-ceramic composite electrolyte with high ionic conductivity for all-solid-state lithium batteries. J Solid State Electrochem 24(10):2407–2417. https://doi.org/10.1007/s10008-020-04783-z
Yan W, Wei J, Chen T, Duan L, Wang L, Xue X, Jin Z (2021) Superstretchable, thermostable and ultrahigh-loading lithium–sulfur batteries based on nanostructural gel cathodes and gel electrolytes. Nano Energy. https://doi.org/10.1016/j.nanoen.2020.105510
Chen T, Kong W, Zhang Z, Wang L, Hu Y, Zhu G, Jin Z (2018) Ionic liquid-immobilized polymer gel electrolyte with self-healing capability, high ionic conductivity and heat resistance for dendrite-free lithium metal batteries. Nano Energy 54:17–25. https://doi.org/10.1016/j.nanoen.2018.09.059
Wei J, Zhang P, Shen T, Liu Y, Dai T, Tie Z, Jin Z (2023) Supramolecule-based excluded-volume electrolytes and conjugated sulfonamide cathodes for high-voltage and long-cycling aqueous zinc-ion batteries. ACS Energy Lett 8(1):762–771. https://doi.org/10.1021/acsenergylett.2c02646
Wei J, Zhang P, Liu Y, Zhu M, Dai T, Tie Z, Jin Z (2023) Wide-voltage-window amphiphilic supramolecule excluded-volume electrolytes for ultra-durable full-cell aqueous potassium-Ion batteries. Chem Eng J 459:141623. https://doi.org/10.1016/j.cej.2023.141623
Yao Y, Liu Z, Wang X, Chen J, Wang X, Wang D, Mao Z (2021) Promoted ion conductivity of sodium salt–poly(ethylene oxide) polymer electrolyte induced by adding conductive beta-alumina and application in all-solid-state sodium batteries. J Mater Sci 56(16):9951–9960
Fertig MP, Skadell K, Schulz M, Dirksen C, Adelhelm P, Stelter M (2022) From high- to low-temperature: the revival of sodium-beta alumina for sodium solid-state batteries. Batteries Supercaps 5(1):1–26. https://doi.org/10.1002/batt.202100131
Acknowledgements
We are highly thankful to the Central Instrumentation Facility (CIF) of Lovely Professional University to provide the XRD, FESEM, TGA, and Electrochemical Workstation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amin, O., Sinha, S., Maji, P.S. et al. Effect of indium doping on thermal stability and dielectric property in sodium beta alumina solid electrolyte. J Solid State Electrochem 27, 2387–2394 (2023). https://doi.org/10.1007/s10008-023-05523-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05523-9