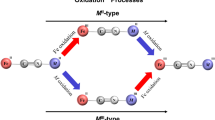
Abstract
A Prussian blue analogue (Co,Fe)CN with Fe and Co ions linked by CN– ions was synthesized; the synthesis was aimed at obtaining cobalt hexacyanoferrate (CoHCF). However, X-ray phase analysis showed the resemblance of (Co,Fe)CN to sodium iron hexacyanocobaltate (FeHCC) Na0.108Fe[Co(CN)6]. The electron paramagnetic resonance (EPR) spectra do not agree with the transition of CoHCF to the latter compound, but do not exclude the possibility of only partial re-coordination of the cyanide ions. At the same time, EPR also excludes formation of the targeted CoHCF. The Fourier transform infrared spectra also indicate that (Co,Fe)CN differs from CoHCF and FeHCC.
The (Co,Fe)CN electrochemistry has been studied by cyclic voltammetry. (Co,Fe)CN is electroactive in solutions of alkali metal salts, from Li to Cs, and ammonium salts. The shape of (Co,Fe)CN cyclic voltammograms is unique and does not resemble the curves of CoHCF and FeHCC. It should be noted that the electrode process of CoHCF is blocked in solutions of rubidium, cesium, and ammonium salts, while (Co,Fe)CN is electroactive in these solutions. (Co,Fe)CN produces one pair of peaks in a heavy alkali metal salt solution, whereas in the presence of light alkali metals, it produces two to three pairs of peaks. Electrochemical data also reveal the difference between (Co,Fe)CN and CoHCF and are one more argument in favor of the partial CN– ion linkage isomerization. Unfortunately, all the evidence for the re-coordination of cyanide ions gained in this work is only indirect.
Graphical Abstract












Similar content being viewed by others
References
Wessells CD, Peddada SV, Huggins RA, Cui Y (2011) Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett 11:5421–5425. https://doi.org/10.1021/nl203193q
Pasta M, Wang RY, Ruffo R, Qiao R, Lee H-W, Shyam B, Guo M, Wang Y, Wray LA, Yang W, Toney MF, Cui Y (2016) Manganese–cobalt hexacyanoferrate cathodes for sodium-ion batteries. J Mater Chem A 4:4211–4223. https://doi.org/10.1039/C5TA10571D
Zhang X, Tao L, He P, Zhang X, He M, Dong F, He S, Li C, Liu H, Wang S, Zhang Y (2018) A novel cobalt hexacyanoferrate/multi-walled carbon nanotubes nanocomposite: spontaneous assembly synthesis and application as electrode materials with significantly improved capacitance for supercapacitors. Electrochim Acta 259:793–802. https://doi.org/10.1016/j.electacta.2017.11.007
Li W-J, Han C, Cheng G, Chou S-L, Liu H-K, Dou S-X (2019) Chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 15:1900470. https://doi.org/10.1002/smll.201900470
Marzak P, Kosiahn M, Yun J, Bandarenka AS (2019) Intercalation of Mg2+ into electrodeposited Prussian blue analogue thin films from aqueous electrolytes. Electrochim Acta 307:157–163. https://doi.org/10.1016/j.electacta.2019.03.094
Ma L, Li X, Zhang G, Huang Z, Han C, Li H, Tang Z, Zhi C (2020) Initiating a wearable solid-state Mg hybrid ion full battery with high voltage, high capacity and ultra-long lifespan in air. Energy Storage Mater 31:451–458. https://doi.org/10.1016/j.ensm.2020.08.001
Goda ES, Lee S, Sohail M, Yoon KR (2020) Prussian blue and its analogues as advanced supercapacitor electrodes. J Energy Chem 50:206–229. https://doi.org/10.1016/j.jechem.2020.03.031
Ru Y, Zheng S, Xue H, Pang H (2020) Potassium cobalt hexacyanoferrate nanocubic assemblies for high-performance aqueous aluminum ion batteries. Chem Eng J 382:122853. https://doi.org/10.1016/j.cej.2019.122853
Zhou A, Cheng W, Wang W, Zhao Q, Xie J, Zhang W, Gao H, Xue L, Li J (2021) Hexacyanoferrate-type Prussian blue analogs: principles and advances toward high-performance sodium and potassium ion batteries. Adv Energy Mater 11:2000943. https://doi.org/10.1002/aenm.202000943
Peng J, Zhang W, Liu Q, Wang J, Chou S, Liu H, Dou S (2022) Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv Mater 34:2108384. https://doi.org/10.1002/adma.202108384
Xie B, Sun B, Gao T, Ma Y, Yin G, Zuo P (2022) Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord Chem Rev 460:214478. https://doi.org/10.1016/j.ccr.2022.214478
Wang Q, Li J, Jin H, Xin S, Gao H (2022) Prussian-blue materials: revealing new opportunities for rechargeable batteries. InfoMat 4:e12311. https://doi.org/10.1002/inf2.12311
Yang Y, Zhou J, Wang L, Jiao Z, Xiao M, Huang Q, Liu M, Shao Q, Sun X, Zhang J (2022) Prussian blue and its analogues as cathode materials for Na-, K-, Mg-, Ca-, Zn- and Al-ion batteries. Nano Energy 99:107424. https://doi.org/10.1016/j.nanoen.2022.107424
You Y, Wu X-L, Yin Y-X, Guo Y-G (2014) High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ Sci 7:1643–1647. https://doi.org/10.1039/C3EE44004D
Zhang Y, Wang Y, Lu L, Sun C, Yu DYW (2021) Vanadium hexacyanoferrate with two redox active sites as cathode material for aqueous Zn-ion batteries. J Power Sources 484:229263. https://doi.org/10.1016/j.jpowsour.2020.229263
Kulesza PJ, Malik MA, Zamponi S, Berrettoni M, Marassi R (1995) Electrolyte-cation-dependent coloring, electrochromism and thermochromism of cobalt(II) hexacyanoferrate(III, II) films. J Electroanal Chem 397:287–292. https://doi.org/10.1016/0022-0728(95)04187-8
Kulesza PJ, Malik MA, Miecznikowski K, Wolkiewicz A, Zamponi S, Berrettoni M, Marassi R (1996) Countercation-sensitive electrochromism of cobalt hexacyanoferrate films. J Electrochem Soc 143:L10–L12. https://doi.org/10.1149/1.1836374
Kulesza PJ, Malik MA, Berrettoni M, Giorgetti M, Zamponi S, Schmidt R, Marassi R (1998) Electrochemical charging, countercation accommodation, and spectrochemical identity of microcrystalline solid cobalt hexacyanoferrate. J Phys Chem B 102:1870–1876. https://doi.org/10.1021/jp9726495
Kulesza PJ, Zamponi S, Malik MA, Berrettoni M, Wolkiewicz A, Marassi R (1998) Spectroelectrochemical characterization of cobalt hexacyanoferrate films in potassium salt electrolyte. Electrochim Acta 43:919–923. https://doi.org/10.1016/S0013-4686(97)00212-0
Chen S-M (1998) Characterization and electrocatalytic properties of cobalt hexacyanoferrate films. Electrochim Acta 43:3359–3369. https://doi.org/10.1016/S0013-4686(98)00074-7
Kaplun MM, Ivanov VD (2000) Modification of platinum and graphite electrodes by cobalt hexacyanoferrate films. Russ J Electrochem 36:501–508. https://doi.org/10.1007/BF02757413
Kaplun MM, Smirnov YE, Mikli V, Malev VV (2001) Structure of cobalt hexacyanoferrate films synthesized from a complex electrolyte. Russ J Electrochem 37:914–924. https://doi.org/10.1023/A:1011992109433
Ivanov VD, Kaplun MM, Kondrat’ev VV, Tikhomirova AV, Zigel’ VV, Yakovleva SV, Malev VV (2002) Charge transfer in iron and cobalt hexacyanoferrate films: effect of the film thickness and the counterion nature. Russ J Electrochem 38:173–181. https://doi.org/10.1023/A:1016820332194
Vittal R, Gomathi H (2002) Beneficial effects of cetyltrimethylammonium bromide in the modification of electrodes with cobalt hexacyanoferrate surface films. J Phys Chem B 106:10135–10143. https://doi.org/10.1021/jp020337i
Lezna RO, Romagnoli R, de Tacconi NR, Rajeshwar K (2002) Cobalt hexacyanoferrate: compound stoichiometry, infrared spectroelectrochemistry, and photoinduced electron transfer. J Phys Chem B 106:3612–3621. https://doi.org/10.1021/jp013991r
Shi L-H, Wu T, Wang M-J, Li D, Zhang Y-J, Li J-H (2005) Molecule-based cobalt hexacyanoferrate nanoparticle: synthesis, characterization, and its electrochemical properties. Chin J Chem 23:149–154. https://doi.org/10.1002/cjoc.200590149
Berrettoni M, Giorgetti M, Zamponi S, Conti P, Ranganathan D, Zanotto A, Saladino ML, Caponetti E (2010) Synthesis and characterization of nanostructured cobalt hexacyanoferrate. J Phys Chem C 114:6401–6407. https://doi.org/10.1021/jp100367p
Bo AL, Lin XQ (1999) In-situ external reflection FTIR spectroelectrochemical and XPS investigations of cobalt-cyanometallates as inorganic polymeric materials on a platinum electrode. Talanta 49:717–723. https://doi.org/10.1016/S0039-9140(99)00014-4
Sauter S, Wittstock G, Szargan R (2001) Localisation of electrochemical oxidation processes in nickel and cobalt hexacyanoferrates investigated by analysis of the multiplet patterns in X-ray photoelectron spectra. Phys Chem Chem Phys 3:562–569. https://doi.org/10.1039/b008430l
Hallmeier KH, Sauter S, Szargan R (2001) XANES and EXAFS investigations of bonding and structure of Ni and Co derivatives from Prussian blue coordination compounds. Inorg Chem Commun 4:153–156. https://doi.org/10.1016/S1387-7003(01)00151-4
Hillman AR, Skopek MA, Gurman SJ (2010) EXAFS structural studies of electrodeposited Co and Ni hexacyanoferrate films. J Solid State Electrochem 14:1997–2010. https://doi.org/10.1007/s10008-010-1033-9
Hosseini P, Wittstock G, Brand I (2018) Infrared spectroelectrochemical analysis of potential dependent changes in cobalt hexacyanoferrate and copper hexacyanoferrate films on gold electrodes. J Electroanal Chem 812:199–206. https://doi.org/10.1016/j.jelechem.2017.12.029
Sato O, Einaga Y, Iyoda T, Fujishima A, Hashimoto K (1997) Cation-driven electron transfer involving a spin transition at room temperature in a cobalt iron cyanide thin film. J Phys Chem B 101:3903–3905. https://doi.org/10.1021/jp9701451
Sato O, Iyoda T, Fujishima A, Hashimoto K (1996) Photoinduced magnetization of a cobalt-iron cyanide. Science 272:704–705. https://doi.org/10.1126/science.272.5262.704
Sato O, Einaga Y, Fujishima A, Hashimoto K (1999) Photoinduced long-range magnetic ordering of a cobalt-iron cyanide. Inorg Chem 38:4405–4412. https://doi.org/10.1021/ic980741p
Goujon A, Roubeau O, Varret F, Dolbecq A, Bleuzen A, Verdaguer M (2000) Photo-excitation from dia- to ferri-magnetism in a Rb–Co–hexacyanoferrate Prussian blue analogue. Eur Phys J B 14:115–124. https://doi.org/10.1007/s100510050112
Bleuzen A, Escax V, Ferrier A, Villain F, Verdaguer M, Münsch P, Itié J-P (2004) Thermally induced electron transfer in a CsCoFe Prussian blue derivative: the specific role of the alkali-metal ion. Angew Chem Int Ed 43:3728–3731. https://doi.org/10.1002/anie.200460086
Giorgetti M, Aquilanti G, Ciabocco M, Berrettoni M (2015) Anatase-driven charge transfer involving a spin transition in cobalt iron cyanide nanostructures. Phys Chem Chem Phys 17:22519–22522. https://doi.org/10.1039/C5CP03580E
Bordage A, Bleuzen A (2020) Influence of the number of alkali cation on the photo-induced CoIIIFeII ↔ CoIIFeIII charge transfer in CsxCoFe PBAs – A Co K-edge XANES study. Radiat Phys Chem 175:108143. https://doi.org/10.1016/j.radphyschem.2019.02.002
Cammarata M, Zerdane S, Balducci L, Azzolina G, Mazerat S, Exertier C, Trabuco M, Levantino M, Alonso-Mori R, Glownia JM, Song S, Catala L, Mallah T, Matar SF, Collet E (2021) Charge transfer driven by ultrafast spin transition in a CoFe Prussian blue analogue. Nat Chem 13:10–14. https://doi.org/10.1038/s41557-020-00597-8
Moon SB, Moon JD (1995) Electrochemistry and electrokinetics of Prussian blue modified electrodes obtained using Fe(III) complex. Bull Korean Chem Soc 16:819–823. https://doi.org/10.5012/bkcs.1995.16.9.819
Shriver DF, Shriver SA, Anderson SE (1965) Ligand field strength of the nitrogen end of cyanide and structures of cubic cyanide polymers. Inorg Chem 4:725–730. https://doi.org/10.1021/ic50027a028
House JE Jr, Bailar JC Jr (1969) Kinetics of the linkage isomerization in iron(II) hexacyanochromate(III). Inorg Chem 8:672–673. https://doi.org/10.1021/ic50073a051
Cosmano RJ, House JE Jr (1975) Thermally induced linkage isomerization in KCd[Fe(CN)6]. Thermochim Acta 13:127–131. https://doi.org/10.1016/0040-6031(75)80075-X
House JE Jr, Kob NE (1993) Linkage isomerization in potassium cadmium hexacyanoferrate induced by ultrasound. Inorg Chem 32:1053–1054. https://doi.org/10.1021/ic00058a052
Reguera E, Bertrán JA, Nuñez L (1994) Study of the linkage isomerization process in hexacyanometallates. Polyhedron 13:1619–1624. https://doi.org/10.1016/S0277-5387(00)83457-9
Dostal A, Schroeder U, Scholz F (1995) Electrochemistry of chromium(II) hexacyanochromate(III) and electrochemically induced isomerization of solid iron(II) hexacyanochromate(III) mechanically immobilized on the surface of a graphite electrode. Inorg Chem 34:1711–1717. https://doi.org/10.1021/ic00111a017
Coronado E, Giménez-López MC, Levchenko G, Romero FM, García-Baonza V, Milner A, Paz-Pasternak M (2005) Pressure-tuning of magnetism and linkage isomerism in iron(II) hexacyanochromate. J Am Chem Soc 127:4580–4581. https://doi.org/10.1021/ja043166z
Dumont MF, Risset ON, Knowles ES, Yamamoto T, Pajerowski DM, Meisel MW, Talham DR (2013) Synthesis and size control of iron(II) hexacyanochromate(III) nanoparticles and the effect of particle size on linkage isomerism. Inorg Chem 52:4494–4501. https://doi.org/10.1021/ic302764k
Wilamowska M, Lisowska-Oleksiak A (2011) Synthesis and electrochemical characterization of poly(3,4-ethylenedioxythiophene) modified by iron hexacyanocobaltate. Solid State Ion 188:118–123. https://doi.org/10.1016/j.ssi.2010.09.037
Yamada Y, Yoneda M, Fukuzumi S (2013) A robust one-compartment fuel cell with a polynuclear cyanide complex as a cathode for utilizing H2O2 as a sustainable fuel at ambient conditions. Chem Eur J 19:11733–11741. https://doi.org/10.1002/chem.201300783
Ciabocco M, Berrettoni M, Chillura DFM, Giorgetti M (2014) Electrochemistry of TiO2–iron hexacyanocobaltate composite electrodes. Solid State Ion 259:53–58. https://doi.org/10.1016/j.ssi.2014.02.019
Mullaliu A, Conti P, Aquilanti G, Plaisier JR, Stievano L, Giorgetti M (2018) Operando XAFS and XRD study of a Prussian blue analogue cathode material: iron hexacyanocobaltate. Condens Matter 3:36. https://doi.org/10.3390/condmat3040036
Zhang K, Varma RS, Jang HW, Choi J-W, Shokouhimehr M (2019) +Iron hexacyanocobaltate metal-organic framework: highly reversible and stationary electrode material with rich borders for lithium-ion batteries. J Alloys Compd 791:911–917. https://doi.org/10.1016/j.jallcom.2019.03.379
Pramudita JC, Schmid S, Godfrey T, Whittle T, Alam M, Hanley T, Brand HEA, Sharma N (2014) Sodium uptake in cell construction and subsequent in operando electrode behaviour of Prussian blue analogues, Fe[Fe(CN)6]1–x·yH2O and FeCo(CN)6. Phys Chem Chem Phys 16:24178–24187. https://doi.org/10.1039/c4cp02676d
Dostal A, Meyer B, Scholz F, Schroeder U, Bond AM, Marken F, Shaw SJ (1995) Electrochemical study of microcrystalline solid Prussian blue particles mechanically attached to graphite and gold electrodes: electrochemically induced lattice reconstruction. J Phys Chem 99:2096–2103. https://doi.org/10.1021/j100007a045
Reguera E, Bertrán J, Diaz C, Blanco J, Rondón S (1990) Mössbauer and infrared spectroscopic studies of novel mixed valence states in cobaltous ferrocyanides and ferricyanides. Hyperfine Interact 53:391–395. https://doi.org/10.1007/BF02101072
Lejeune J, Brubach J-B, Roy P, Bleuzen A (2014) Application of the infrared spectroscopy to the structural study of Prussian blue analogues. C R Chimie 17:534–540. https://doi.org/10.1016/j.crci.2014.01.017
Mullica DF, Oliver JD, Milligan WO, Hills FW (1979) Ferrous hexacyanocobaltate dodecahydrate. Inorg Nucl Chem Lett 15:361–365. https://doi.org/10.1016/0020-1650(79)80111-7
Joseph G, Gomathi H, Rao GP (1991) Electrodes modified with cobalt hexacyanoferrate. J Electroanal Chem 304:263–269. https://doi.org/10.1016/0022-0728(91)85509-N
Jiang M, Zhou X, Zhao Z (1991) Cobalt(II) - cyanometallates as new inorganic polymeric materials for surface-modification of some conducting substrates. Ber Bunsenges Phys Chem 95:720–727. https://doi.org/10.1002/bbpc.19910950611
Ivanov VD, Alieva AR (2000) Electrochemical behavior of electrode modified with a cobalt hexacyanoferrate film: effect of the supporting electrolyte cation. Rus J Electrochem 36:852–860. https://doi.org/10.1007/BF02757058
Canto-Aguilar EJ, Oliver-Tolentino MA, Ramos-Sánchez G, González I (2021) Effect of the external metal on the electrochemical behavior of M3[Co(CN)6]2 (M: Co, Ni, Cu, Zn), towards their use as anodes in potassium ion batteries. Electrochim Acta 371:137828. https://doi.org/10.1016/j.electacta.2021.137828
Yang C-F, Wang Q, Yi C-Y, Zhao J-H, Fang J, Shen W-G (2012) Electrochemical properties of nanostructured cobalt hexacyanoferrate containing K+ and Cs+ synthesized in water-in-oil AOT reverse microemulsions. J Electroanal Chem 674:30–37. https://doi.org/10.1016/j.jelechem.2012.04.007
Acknowledgements
The author is grateful to Dr. Mikhail Yu. Skripkin for the fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ivanov, V.D. Electrochemical properties of (Co,Fe)CN, cobaltous Prussian blue analogue. J Solid State Electrochem 27, 2419–2432 (2023). https://doi.org/10.1007/s10008-023-05519-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05519-5