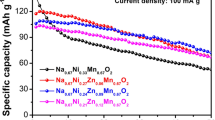
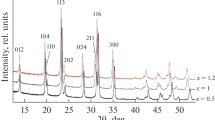
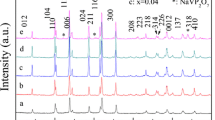
Abstract
Solid-state synthesis of novel electrode materials of P2-type layered oxides Na0.67Ni0.33-xVxMn0.67O2 (where x = 0, 0.05, and 0.11) for the application as cathode materials in rechargeable non-aqueous sodium-ion batteries is reported. Na0.67Ni0.22V0.11Mn0.67O2 electrode delivers significantly improved capacity retention than the pristine P2-Na0.67Ni0.33Mn0.67O2 when cycled between 1.5 and 4.3 V. The effect of organic ester electrolytes with 1.0 M NaClO4 shows diverse effects in the cathode material. The best capacity retention for V-substituted electrodes is observed in ethylene carbonate (EC): propylene carbonate (PC): dimethyl carbonate (DMC) (0.45: 0.45: 0.1) with 1.0 M NaClO4 salt at a C-rate of 0.2C among four different ester electrolytes investigated [PC, EC: PC (1: 1), EC: DEC (1: 1), and EC: PC: DMC (0.45: 0.45: 0.1)].
Graphical Abstract








Similar content being viewed by others
Abbreviations
- NNMO:
-
Sodium nickel manganese oxide
- NVMO:
-
Vanadium substituted NNMO
- SEI:
-
Solid electrolyte interphase
- EC:
-
Ethylene carbonate
- DEC:
-
Diethylene carbonate
- PC:
-
Propylene carbonate
- FEC:
-
Fluroethylene carbonate
- DMC:
-
Dimethyl carbonate
- XRD:
-
X-ray diffraction
- EPR:
-
Electron paramagnetic resonance
- PVDF:
-
Poly(vinylidene fluoride)
- NMP:
-
N-Methyl-2-pyrrolidone
- SEM:
-
Scanning electron microscopy
- EDX:
-
Energy-dispersive X-ray
- CV:
-
Cyclic voltammetry
- DFT:
-
Density functional theory
- MSD:
-
Mean square displacement
- AIMD:
-
Ab initio molecular dynamics
References
Su H, Jaffer S, Yu H (2016) Transition metal oxides for sodium-ion batteries. Energy Storage Mater, Elsevier 5:116–131. https://doi.org/10.1016/j.ensm.2016.06.005
Palomares V, Casas-Cabanas M, Castillo-Martínez E, Han MH, Rojo T (2013) Update on Na-based battery materials. A growing research path. Energy Environ Sci 6:2312–2337. https://doi.org/10.1039/c3ee41031e
Pan H, Hu YS, Chen L (2013) Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci 6:2338–2360. https://doi.org/10.1039/c3ee40847g
Wang S, Sun C, Wang N, Zhang Q (2019) Ni- and/or Mn-based layered transition metal oxides as cathode materials for sodium ion batteries: status, challenges and countermeasures. J Mater Chem A, Royal Soc Chem 7:10138–10158. https://doi.org/10.1039/c8ta12441h
Delmas C, Fouassier C, Hagenmuller P (1980) Structural classification and properties of the layered oxides. Physica B+C, 99:81–85. https://doi.org/10.1016/0378-4363(80)90214-4
Didier C, Guignard M, Denage C, Szajwaj O, Ito S, Saadoune I, Darriet J, Delmas C (2011) Electrochemical Na-deintercalation from NaVO2. Electrochem Solid-State Lett 14:1–5. https://doi.org/10.1149/1.3555102
Hamani D, Ati M, Tarascon JM, Rozier P (2011) NaxVO2 as possible electrode for Na-ion batteries. Electrochem Commun 13:938–941. https://doi.org/10.1016/j.elecom.2011.06.005
Didier C, Guignard M, Darriet J, Delmas C (2012) O′3−NaxVO2 system: a superstructure for Na1/2VO2. Inorg Chem 51:11007–11016. https://doi.org/10.1021/ic301505e
Guignard M, Didier C, Darriet J, Bordet P, Elkaïm E, Delmas C (2013) P2-Na x VO 2 system as electrodes for batteries and electron-correlated materials. Nat Mater Nat Publish Group 12:74–80. https://doi.org/10.1038/nmat3478
Peng B, Sun Z, Zhao L, Zeng S, Zhang G (2021) Shape-induced kinetics enhancement in layered P2-Na 0.67 Ni 0.33 Mn 0.67 O 2 porous microcuboids enables high energy/power sodium-ion full battery. Batteries Supercaps 4:456–463. https://doi.org/10.1002/batt.202000226
Xu J, Lee DH, Clement R, Leskes M, Pell A, Pintacuda G, Yang X, Grey C, Meng Y (2014) Identifying the critical role of Li substitution in P2-Nax(LiyNixMn1-y-z)O2(0 less than x, y, z less than 1) intercalation cathode materials for high energy Na-ion batteries. Chem Mater 26:1260–1269
Liu K, Tan S, Moon J, Jafta CJ, Li C, Kobayashi T, Lyu H, Bridges CA, Men S, Guo W, Sun Y, Zhang J, Paranthaman MP, Sun XG, Dai S (2020) Insights into the enhanced cycle and rate performances of the F-substituted P2-type oxide cathodes for sodium-ion batteries. Adv Energy Mater 10:1–11. https://doi.org/10.1002/aenm.202000135
Wang P-F, You Y, Yin Y-X, Wang Y-S, Wan L-J, Gu L, Guo Y-G (2016) Suppressing the P2–O2 phase transition of Na 0.67 Mn 0.67 Ni 0.33 O 2 by magnesium substitution for improved sodium-ion batteries. Angew Chem 128:7571–7575. https://doi.org/10.1002/ange.201602202
Peng B, Sun Z, Zhao L, Li J, Zhang G (2021) Dual-manipulation on P2-Na0.67Ni0.33Mn0.67O2 layered cathode toward sodium-ion full cell with record operating voltage beyond 3. 5 V. Energy Storage Mater Elsevier B. V 35:620–629. https://doi.org/10.1016/j.ensm.2020.11.037
Wu X, Guo J, Wang D, Zhong G, McDonald M.J, Yang Y (2015) P2-type Na0.66Ni0.33-XZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. J Power Sourc Elsevier B.V 281:18–26. https://doi.org/10.1016/j.jpowsour.2014.12.083
Peng B, Chen Y, Wang F, Sun Z, Zhao L, Zhang X, Wang W, Zhang G (2022) Unusual site‐selective doping in layered cathode strengthens electrostatic cohesion of alkali‐metal layer for practicable sodium‐ion full cell. Advanced Materials 34(6):2103210. https://doi.org/10.1002/adma.202103210
Kubota K, Yoda Y, Komaba S (2017) Origin of enhanced capacity retention of P2-type Na 2/3 Ni 1/3- x Mn 2/3 Cu x O 2 for Na-ion batteries. J Electrochem Soc 164:A2368–A2373. https://doi.org/10.1149/2.0311712jes
Li J, Wang J, He X, Zhang L, Senyshyn A, Yan B, Muehlbauer M, Cao X, Vortmann-Westhoven B, Kraft V, Liu H, Luerenbaum C, Schumacher G, Paillard E, Winter M, Li J (2019) P2 – type Na0.67Mn0.8Cu0.1Mg0.1O2 as a new cathode material for sodium-ion batteries: insights of the synergetic effects of multi-metal substitution and electrolyte optimization. Journal of Power Sourc Elsevier 416:184–192. https://doi.org/10.1016/j.jpowsour.2019.01.086
Yuan D, Hu X, Qian J, Pei F, Wu F, Mao R, Ai X, Yang H, Cao Y (2014) P2-type Na0.67Mn0.65Fe0.2Ni 0.15O2 cathode material with high-capacity for sodium-ion battery. Electrochimica Acta, Elsevier Ltd 116:300–305. https://doi.org/10.1016/j.electacta.2013.10.211
Li ZY, Zhang J, Gao R, Zhang H, Hu Z, Liu X (2016) Unveiling the role of Co in improving the high-rate capability and cycling performance of layered Na0.7Mn0.7Ni0.3-XCoxO2 cathode materials for sodium-ion batteries. ACS Appl Mater Interfaces 8:15439–15448. https://doi.org/10.1021/acsami.6b04073
Zhao W, Tanaka A, Momosaki K, Yamamoto S, Zhang F, Guo Q, Noguchi H (2015) Enhanced electrochemical performance of Ti substituted P2-Na2/3Ni1/4Mn3/4O2 cathode material for sodium ion batteries. Electrochimica Acta, Elsevier Ltd 170:171–181. https://doi.org/10.1016/j.electacta.2015.04.125
Huang Y, Yan Z, Luo W, Hu Z, Liu G, Zhang L, Yang X, Ou M, Liu W, Huang L, Lin H, Chen CT, Luo J, Li S, Han J, Chou S, Huang Y (2020) Vitalization of P2–Na2/3Ni1/3Mn2/3O2 at high-voltage cyclability via combined structural modulation for sodium-ion batteries. Energy Storage Mater 29:182–189. https://doi.org/10.1016/j.ensm.2020.04.012
Li J, Risthaus T, Wang J, Zhou D, He X, Ehteshami N, Murzin V, Friesen A, Liu H, Hou X, Diehl M, Paillard E, Winter M, Li J (2020) The effect of Sn substitution on the structure and oxygen activity of Na0.67Ni0.33Mn0.67O2 cathode materials for sodium ion batteries. J Power Sourc Elsevier B.V., 449:227554. https://doi.org/10.1016/j.jpowsour.2019.227554
Chagas LG, Buchholz D, Vaalma C, Wuab L, Passerini S (2014) P-type NaxNi0.22Co0.11Mn0.66O2 materials: linking synthesis with structure and electrochemical performance. J Mater Chemi A 2:20263–20270. https://doi.org/10.1039/c4ta03946g
Kataoka R, Mukai T, Yoshizawa A, Inoue K, Kiyobayashi T, Sakai T (2015) High capacity positive electrode material for room temperature Na ion battery: Na x Mn 2/3 Co 1/6 Ni 1/6 O 2. J Electrochem Soc 162:A553–A558. https://doi.org/10.1149/2.0181504jes
Zheng L, Li J, Obrovac MN (2017) Crystal structures and electrochemical performance of air-stable Na2/3Ni1/3-XCuxMn2/3O2 in sodium cells. Chem Mater 29:1623–1631. https://doi.org/10.1021/acs.chemmater.6b04769
Pahari D, Puravankara S (2020) On controlling the P2-O2 phase transition by optimal Ti-substitution on Ni- site in P2-Type Na0.67Ni0.33Mn0.67O2 (NNMO) cathode for Na-ion batteries. J Power Sourc, Elsevier B.V 455:227957. https://doi.org/10.1016/j.jpowsour.2020.227957
Kim D, Lee E, Slater M, Lu W, Rood S, Johnson CS (2012) Layered Na[Ni 1/3Fe 1/3Mn 1/3]O 2 cathodes for Na-ion battery application. Electrochem Commun Elsevier B.V 18:66–69. https://doi.org/10.1016/j.elecom.2012.02.020
Yoshida H, Yabuuchi N, Kubota K, Ikeuchi I, Garsuch A, Schulz-Dobrick M, Komaba S (2014) P2-Type Na2/3Ni1/3Mn2/3−xTixO2 as a new positive electrode for higher energy Na-ion batteries. Chem Commun 50:3677–3680. https://doi.org/10.1039/c3cc49856e
Bommier C, Ji X (2018) Electrolytes, SEI formation, and binders: a review of nonelectrode factors for sodium-ion battery anodes. Small 14:1–20. https://doi.org/10.1002/smll.201703576
Ponrouch A, Marchante E, Courty M, Tarascon JM, Palacín MR (2012) In search of an optimized electrolyte for Na-ion batteries. Energy Environ Sci 5:8572–8583. https://doi.org/10.1039/c2ee22258b
Sun Y, Shi P, Xiang H, Liang X, Yu Y (2019) High-safety nonaqueous electrolytes and interphases for sodium-ion batteries. Small 15:1–17. https://doi.org/10.1002/smll.201805479
Li Q, Chen J, Fan L, Kong X, Lu Y (2016) Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ 1:18–42. https://doi.org/10.1016/j.gee.2016.04.006
Ponrouch A, Dedryvère R, Monti D, Demet AE, Ateba Mba JM, Croguennec L, Masquelier C, Johansson P, Palacín MR (2013) Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ Sci 6:2361–2369. https://doi.org/10.1039/c3ee41379a
Lu Z, Dahn JR (2001) In situ X-ray diffraction study of P2-Na[Sub 2/3][Ni[Sub 1/3]Mn[Sub 2/3]]O[Sub 2]. J Electrochem Soc 148:A1225. https://doi.org/10.1149/1.1407247
Che H, Chen S, Xie Y, Wang H, Amine K, Liao XZ, Ma ZF (2017) Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ Sci, Royal Soc Chem 10:1075–1101. https://doi.org/10.1039/c7ee00524e
Liu Y, Fang X, Zhang A, Shen C, Liu Q, Enaya HA, Zhou C (2016) Layered P2-Na2/3[Ni1/3Mn2/3]O2 as high-voltage cathode for sodium-ion batteries: the capacity decay mechanism and Al2O3 surface modification. Nano Energy, Elsevier 27:27–34. https://doi.org/10.1016/j.nanoen.2016.06.026
Wang H, Yang B, Liao XZ, Xu J, Yang D, He YS, Ma ZF (2013) Electrochemical properties of P2-Na2/3[Ni1/3Mn 2/3]O2 cathode material for sodium ion batteries when cycled in different voltage ranges. Electrochimica Acta, Elsevier Ltd 113:200–204. https://doi.org/10.1016/j.electacta.2013.09.098
Lee SY, Kim JH, Kang YC (2017) Electrochemical properties of P2-type Na2/3Ni1/3Mn2/3O2 plates synthesized by spray pyrolysis process for sodium-ion batteries. Electrochimica Acta, Elsevier Ltd 225:86–92. https://doi.org/10.1016/j.electacta.2016.11.141
Ponrouch A, Monti D, Boschin A, Steen B, Johansson P, Palacín MR (2015) Non-aqueous electrolytes for sodium-ion batteries. J Mater Chem A, Royal Soc Chem 3:22–42. https://doi.org/10.1039/c4ta04428b
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B Condens Matter Mater Phys 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50. https://doi.org/10.1016/0927-0256(96)00008-0
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Okhotnikov K, Charpentier T, Cadars S (2016) Supercell program: a combinatorial structure-generation approach for the local-level modeling of atomic substitutions and partial occupancies in crystals. J Cheminform 8:17. https://doi.org/10.1186/s13321-016-0129-3
Henkelman G, Arnaldsson A, Jónsson H (2006) A fast and robust algorithm for Bader decomposition of charge density. Comput Mater Sci 36:354–360. https://doi.org/10.1016/j.commatsci.2005.04.010
Nos´e S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
Sendek AD, Cubuk ED, Antoniuk ER, Cheon G, Cui Y, Reed EJ (2018) Machine learning-assisted discovery of solid Li-ion conducting materials. Chem Mater 31:342–352
Lee DH, Xu J, Meng YS (2013) An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys Chem Chem Phys 15:3304–3312. https://doi.org/10.1039/c2cp44467d
Kim J, Kwon J, Kim M, Do J, Lee D, Han H (2016) Low-dielectric-constant polyimide aerogel composite films with low water uptake. Polym J 48:829–834. https://doi.org/10.1038/pj.2016.37
Stoyanova R, Zhecheva E, Vassilev S (2006) Mn4+ environment in layered Li[Mg0.5-XNi XMn0.5]O2 oxides monitored by EPR spectroscopy. J Solid State Chem 179:378–388. https://doi.org/10.1016/j.jssc.2005.10.038
Singh G, López Del Amo JM, Galceran M, Pérez-Villar S, Rojo T (2015) Structural evolution during sodium deintercalation/intercalation in Na2/3[Fe1/2Mn1/2]O2. J Mater Chem A 3:6954–6961. https://doi.org/10.1039/c4ta06360k
Iliev MN, Litvinchuk AP, Meng RL, Sun YY, Cmaidalka J, Chu CW (2004) Raman phonons and ageing-related surface disorder in NaxCoO2. Physica C 402:239–242. https://doi.org/10.1016/j.physc.2003.09.085
Gutierrez A, Dose WM, Borkiewicz O, Guo F, Avdeev M, Kim S, Fister TT, Ren Y, Bareño J, Johnson CS (2018) On disrupting the Na+-ion/vacancy ordering in P2-type sodium-manganese-nickel oxide cathodes for Na+-Ion batteries. J Phys Chem C 122:23251–23260. https://doi.org/10.1021/acs.jpcc.8b05537
Manikandan P, Ramasubramonian D, Shaijumon MM (2016) Layered P2-type Na0.5Ni0.25Mn0.75O2 as a high performance cathode material for sodium-ion batteries. Electrochimica Acta, Elsevier Ltd 206:199–206. https://doi.org/10.1016/j.electacta.2016.04.138
Yabuuchi N, Kajiyama M, Iwatate J, Nishikawa H, Hitomi S, Okuyama R, Usui R, Yamada Y, Komaba S (2012) P2-type Nax [Fe1/2 Mn1/2[O2 made from Earth-abundant elements for rechargeable Na batteries. Nat Mater, Nat Publish Group 11:512–517. https://doi.org/10.1038/nmat3309
Talaie E, Duffort V, Smith HL, Fultz B, Nazar LF (2015) Structure of the high voltage phase of layered P2-Na2/3-z[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability. Energy Environ Sci Royal Soc Chem 8:2512–2523. https://doi.org/10.1039/c5ee01365h
Biecher Y, Smiley DL, Guignard M, Fauth F, Berthelot R, Delmas C, Goward GR, Carlier D (2020) Original layered OP4-(Li, Na)XCoO2 phase: insights on its structure, electronic structure, and dynamics from solid state NMR. Inorg Chem 59:5339–5349. https://doi.org/10.1021/acs.inorgchem.9b03417
Clément RJ, Billaud J, Robert Armstrong A, Singh G, Rojo T, Bruce PG, Grey CP (2016) Structurally stable Mg-doped P2-Na2/3Mn1-YMgyO2 sodium-ion battery cathodes with high rate performance: insights from electrochemical, NMR and diffraction studies. Energy Environ Sci 9:3240–3251. https://doi.org/10.1039/c6ee01750a
Wang J, Zhou D, He X, Zhang L, Cao X, Ning D, Yan B, Qi X, Li J, Murzin V, Paillard E, Liu X, Schumacher G, Winter M, Li J (2020) Insights into P2-type layered positive electrodes for sodium batteries: from long: from short-range order. ACS Appl Mater Interfaces 12:5017–5024. https://doi.org/10.1021/acsami.9b18109
Somerville JW, Sobkowiak A, Tapia-Ruiz N, Billaud J, Lozano JG, House RA, Bruce PG (2019) Nature of the ‘“Z”’-phase in layered na-ion battery cathodes. Energy Environ Sci 12:2223–2232. https://doi.org/10.5287/bodleian
Li Y, Yang Z, Xu S, Mu L, Gu L, Hu YS, Li H, Chen L (2015) Air-stable copper-based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a new positive electrode material for sodium-ion batteries. Advanced Science 2(6):1500031. https://doi.org/10.1002/advs.201500031
Billaud J, Singh G, Armstrong AR, Gonzalo E, Roddatis V, Armand M, Rojo T, Bruce PG (2014) Na0.67Mn1-XMgxO2 (0 ≤ x ≤ 0.2): a high capacity cathode for sodium-ion batteries. Energy Environ Sci 7:1387–1391. https://doi.org/10.1039/c4ee00465e
Clément RJ, Bruce PG, Grey CP (2015) Review—manganese-based P2-type transition metal oxides as sodium-ion battery cathode materials. J Electrochem Soc 162:A2589–A2604. https://doi.org/10.1149/2.0201514jes
Komaba S, Murata W, Ishikawa T, Yabuuchi N, Ozeki T, Nakayama T, Ogata A, Gotoh K, Fujiwara K (2011) Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries. Adv Func Mater 21:3859–3867. https://doi.org/10.1002/adfm.201100854
Ponrouch A, Goñi AR, Palacín MR (2013) High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem Commun, Elsevier B.V 27:85–88. https://doi.org/10.1016/j.elecom.2012.10.038
Wang L, Huang KW, Chen J, Zheng J (2019) Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci Adv 5:1–11. https://doi.org/10.1126/sciadv.aax4279
Schon TB, An SY, Tilley AJ, Seferos DS (2019) Unusual capacity increases with cycling for ladder-type microporous polymers. ACS Appl Mater Interfaces 11:1739–1747. https://doi.org/10.1021/acsami.8b18293
Acknowledgements
This work was supported by funding from the Science & Engineering Research Board (SERB), India, Early Career Research Award (Grant No: ECR/2016/002003). DP acknowledges IIT Kharagpur for the Ph.D. fellowship. DP also thanks Late Prof. Sudipto Ghosh, Department of Metallurgical and Materials Engineering, IIT Kharagpur, for help with electrochemical measurements. DD acknowledges CSIR, New Delhi, for the Ph.D. fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pahari, D., Chowdhury, A., Das, D. et al. The evolution of structure–property relationship of P2-type Na0.67Ni0.33Mn0.67O2 by vanadium substitution and organic electrolyte combinations for sodium-ion batteries. J Solid State Electrochem 27, 2067–2082 (2023). https://doi.org/10.1007/s10008-023-05466-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05466-1