Abstract
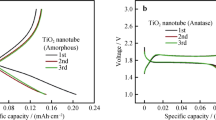
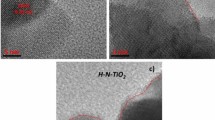
The nanotubular structure of titanium dioxide (TiO2) is most suitable for creating high-performance energy storage and conversion devices. This paper reports on the synthesis of an array of nanotubes (NTs) from TiO2 by electrochemical anodization of titanium sheets using electrolytes based on fluorine and glycerol. The results of SEM and X-ray spectral analysis of the obtained material revealed the anatase phase of TiO2 nanotubes with an inner diameter of 96–150 nm and a length of 0.6 ± 0.1 μm. The electrochemical behavior of the resulting electrode was studied in a solution of Mg(TFSI)2 based on ethylene carbonate/dimethyl carbonate (1/1). From the cyclic voltammograms, the diffusion coefficient and rate constant were determined to be 1.51·10−10 cm2·s−1, k = 1.55·10−10 cm·s−1 (reduction), respectively. The value of the Coulomb efficiency at low discharge current is higher (88%) than at high discharge current (56%). At a high discharge current (1C), it is noticeable that the charge capacity in the cathodic process is much higher than in the anodic process.










Similar content being viewed by others
References
Zhang Y, Geng H, Wei W et al (2019) Challenges and recent progress in the design of advanced electrode materials for rechargeable Mg batteries. Energy Stor Mater 20:118–138
Attias R, Salama M, Hirsch B et al (2019) Anode-electrolyte interfaces in secondary magnesium batteries. Joule 3:27–52
Sheha E, El-Deftar M (2018) Magnesium hexakis(methanol)-dinitrate complex electrolyte for use in rechargeable magnesium batteries. J Solid State Electrochem 22:2671–2679. https://doi.org/10.1007/s10008-018-3986-z
Guo Q, Zeng W, Liu SL et al (2021) Recent developments on anode materials for magnesium-ion batteries: a review. Rare Met 40:290–308
Meng Y, Wang D, Zhao Y et al (2017) Ultrathin TiO2-B nanowires as an anode material for Mg-ion batteries based on a surface Mg storage mechanism. Nanoscale 9:12934–12940. https://doi.org/10.1039/c7nr03493h
Fu Q, Sarapulova A, Trouillet V et al (2019) In operando synchrotron diffraction and in operando X-ray absorption spectroscopy investigations of orthorhombic V2O5 nanowires as cathode materials for Mg-ion batteries. J Am Chem Soc 141:2305–2315. https://doi.org/10.1021/jacs.8b08998
Wang Y, Liu Z, Wang C et al (2018) Highly branched VS4 nanodendrites with 1D atomic-chain structure as a promising cathode material for long-cycling magnesium batteries. Adv Mater 30:1–10. https://doi.org/10.1002/adma.201802563
Yoo HD, Liang Y, Dong H et al (2017) Fast kinetics of magnesium monochloride cations in interlayer-expanded titanium disulfide for magnesium rechargeable batteries. Nat Commun. https://doi.org/10.1038/s41467-017-00431-9
Luo L, Zhou K, Lian R et al (2020) Cation-deficient TiO2(B) nanowires with protons charge compensation for regulating reversible magnesium storage. Nano Energy. https://doi.org/10.1016/j.nanoen.2020.104716
Liu G, Wu HH, Meng Q et al (2020) Role of the anatase/TiO2(B) heterointerface for ultrastable high-rate lithium and sodium energy storage performance. Nanoscale Horizons 5:150–162. https://doi.org/10.1039/c9nh00402e
Minella M, Versaci D, Casino S et al (2017) Anodic materials for lithium-ion batteries: TiO2-rGO composites for high power applications. Electrochim Acta 230:132–140. https://doi.org/10.1016/j.electacta.2017.01.190
Ha SY, Lee YW, Woo SW et al (2014) Magnesium(II) bis(trifluoromethane sulfonyl) imide-based electrolytes with wide electrochemical windows for rechargeable magnesium batteries. ACS Appl Mater Interfaces 6:4063–4073. https://doi.org/10.1021/am405619v
Jay R, Tomich AW, Zhang J et al (2019) Comparative study of Mg(CB 11 H 12) 2 and Mg(TFSI)2 at the magnesium/electrolyte interface. ACS Appl Mater Interfaces 11:11414–11420. https://doi.org/10.1021/acsami.9b00037
Shterenberg I, Salama M, Yoo HD et al (2015) Evaluation of (CF3SO2)2N − (TFSI) based electrolyte solutions for Mg batteries. J Electrochem Soc 162:A7118–A7128. https://doi.org/10.1149/2.0161513jes
Tran TT, Lamanna WM, Obrovac MN (2012) Evaluation of Mg[N(SO2CF3)2]2/acetonitrile electrolyte for use in Mg-ion cells. J Electrochem Soc 159:A2005–A2009. https://doi.org/10.1149/2.012301jes
Son SB, Gao T, Harvey SP et al (2018) An artificial interphase enables reversible magnesium chemistry in carbonate electrolytes. Nat Chem 10:532–539. https://doi.org/10.1038/s41557-018-0019-6
Imamura D, Miyayama M, Hibino M, Kudo T (2003) Mg intercalation properties into V[sub 2]O[sub 5] gel/carbon composites under high-rate condition. J Electrochem Soc 150:A753. https://doi.org/10.1149/1.1571531
Lee SH, DiLeo RA, Marschilok AC et al (2014) Sol gel based synthesis and electrochemistry of magnesium vanadium oxide: a promising cathode material for secondary magnesium ion batteries. ECS Electrochem Lett 3:A87–A90. https://doi.org/10.1149/2.0021408eel
Muldoon J, Bucur CB, Gregory T (2014) Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem Rev 114:11683–11720. https://doi.org/10.1021/cr500049y
Massé RC, Uchaker E, Cao G (2015) Beyond Li-ion: electrode materials for sodium- and magnesium-ion batteries. Sci China Mater 58:715–766. https://doi.org/10.1007/s40843-015-0084-8
Wang C, Huang Y, Lu Y et al (2021) Reversible magnesium metal anode enabled by cooperative solvation/surface engineering in carbonate electrolytes. Nano-Micro Lett 13:1–11. https://doi.org/10.1007/s40820-021-00716-1
Ellis BL, Knauth P, Djenizian T (2014) Three-dimensional self-supported metal oxides for advanced energy storage. Adv Mater 26:3368–3397
Plylahan N, Letiche M, Samy Barr MK et al (2015) High energy and power density TiO2 nanotube electrodes for single and complete lithium-ion batteries. J Power Sources 273:1182–1188. https://doi.org/10.1016/j.jpowsour.2014.09.152
Macák JM, Tsuchiya H, Schmuki P (2005) High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chemie - Int Ed 44:2100–2102. https://doi.org/10.1002/anie.200462459
Patil JV, Mali SS, Shaikh JS et al (2019) Electrochemically anodized ultralong TiO2 nanotubes for supercapacitors. J Electron Mater 48:873–878. https://doi.org/10.1007/s11664-018-6797-1
Macak JM, Tsuchiya H, Ghicov A et al (2007) TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci 11:3–18
Roy P, Berger S, Schmuki P (2011) TiO2 nanotubes: synthesis and applications. Angew Chemie - Int Ed 50:2904–2939
Auer A, Kunze-Liebhäuser J (2019) Recent progress in understanding ion storage in self-organized anodic TiO2 nanotubes. Small Methods. https://doi.org/10.1002/smtd.201800385
Xue J, Wang Z, Hu W et al (2019) The surface wettability of TiO2 nanotube arrays: which is more important—morphology or chemical composition? J Porous Mater 26:91–98. https://doi.org/10.1007/s10934-018-0616-1
Kairatovna AA, Khaisa A, Raigul J et al (2021) Preparation and electrochemical characterization of TiO2 as an anode material for magnesium-ion batteries. Bull Karaganda Univ Chem Ser 104:104–116. https://doi.org/10.31489/2021CH4/104-116
Avchukir K, Burkitbayeva BD, Argimbayeva AM et al (2018) The kinetics of indium electroreduction from chloride solutions. Russ J Electrochem 54:1096–1103. https://doi.org/10.1134/S1023193518120042
Li Y, Zuo P, Li R et al (2021) Formation of an artificial Mg2+-permeable interphase on Mg anodes compatible with ether and carbonate electrolytes. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.0c22520
Brezesinski T, Wang J, Polleux J et al (2009) Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors. J Am Chem Soc 131:1802–1809. https://doi.org/10.1021/ja8057309
Chao D, Zhu C, Yang P et al (2016) Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat Commun. https://doi.org/10.1038/ncomms12122
Chao D, Liang P, Chen Z et al (2016) Pseudocapacitive Na-ion storage boosts high rate and areal capacity of self-branched 2D layered metal chalcogenide nanoarrays. ACS Nano 10:10211–10219. https://doi.org/10.1021/acsnano.6b05566
Hu Z, Wang L, Zhang K et al (2014) MoS2 nanoflowers with expanded interlayers as high-performance anodes for sodium-ion batteries. Angew Chemie - Int Ed 53:12794–12798. https://doi.org/10.1002/anie.201407898
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. John Wiley & Sons, New York, 2nd Edition, ISBN: 978–0–471–04372–0
Funding
The work was funded by the Ministry of Education and Science of the Republic of Kazakhstan — AP09260383.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jumanova, R., Rakhymbay, G., Abildina, A. et al. Nanostructured TiO2 as anode material for magnesium-ion batteries. J Solid State Electrochem 27, 223–233 (2023). https://doi.org/10.1007/s10008-022-05307-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05307-7