Abstract
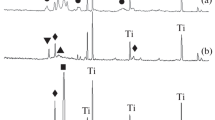
The Ti/TiO2 + ZrO2, Ti/TiO2 + CeOx, and Ti/TiO2 + CeOx + ZrO2 composites, including Y-doped ones, have been fabricated by one-stage plasma electrolytic oxidation (PEO) of titanium in aqueous electrolytes based on Zr(SO4)2 and/or Ce2(SO4)3 with and without additives of Y(CH3COO)2 at effective anodic current density of 0.05 A/cm2 for 10 min. SEM, XRD, and EDX methods have been used for investigation of the composition of the coatings. The resulting PEO coatings contain crystalline TiO2 in rutile and anatase modifications. PEO coatings formed in 0.1 M Zr(SO4)2 electrolyte additionally include TiZrO4 and m-ZrO2. The introduction of yttrium acetate or cerium sulfate into this electrolyte leads to the formation of t-ZrO2 instead of m-ZrO2 in the composition of PEO coatings. The pH sensitivity of obtained composites depends on the composition and morphology of their surface. The Ti/TiO2 + CeOx + ZrO2 composite revealed the highest electrode function slope equal to 51 ± 1 mV/pH and excellent performance for end-point indication potentiometric acid–base titration similar to the conventionally used glass electrode. The Ti/Ce + Zr electrodes have shown excellent performance for determining the alkalinity of both technogenic waters and seawater. These composites are promising for use as wear- and corrosion-resistant pH solid-state sensors for long-term monitoring of highly saline waters, including seawater.










Similar content being viewed by others
References
Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ (2013) Global imprint of climate change on marine life. Nat Clim Change 3:919–925. https://doi.org/10.1038/nclimate1958
Glasgow HB, Burkholder JM, Reed RE, Lewitus AJ, Kleinman E (2004) Real-time remote monitoring of water quality: a review of current applications, and advancements in sensor, telemetry, and computing technologies. J Exp Mar Bio Ecol 300:409–448. https://doi.org/10.1016/j.jembe.2004.02.022
Arefieva O, Nazarkina AV, Gruschakova NV, Skurikhina JE, Kolycheva VB (2019) Impact of mine waters on chemical composition of soil in the Partizansk Coal Basin, Russia. Int Soil Water Conserv Res 7:57–63. https://doi.org/10.1016/j.iswcr.2019.01.001
Fog A, Buck RP (1984) Electronic semiconducting oxides as pH sensors. Sens Actuators 5:137–146. https://doi.org/10.1016/0250-6874(84)80004-9
Manjakkal L, Szwagierczak D, Dahiya R (2020) Metal oxides based electrochemical pH sensors: current progress and future perspectives. Prog Mater Sci 109:100635. https://doi.org/10.1016/j.pmatsci.2019.100635
Gill E, Arshak K, Korostynska O, Arshak A (2008) Mixed metal oxide films as pH sensing materials. Microsyst Technol 14:499–507. https://doi.org/10.1007/s00542-007-0435-9
Zhuiykov S (2012) Solid-state sensors monitoring parameters of water quality for the next generation of wireless sensor networks. Sensor Actuat B-Chem 161:1–20. https://doi.org/10.1016/j.snb.2011.10.078
Manjakkal L, Cvejin K, Bajac B, Kulawik J, Zaraska K, Szwagierczak D (2015) Microstructural, impedance spectroscopic and potentiometric analysis of Ta2O5 electrochemical thick film pH sensors. Electroanalysis 27:1–13. https://doi.org/10.1002/elan.201400571
Chen M, Jin Y, Qu X, Jin Q, Zhao J (2014) Electrochemical impedance spectroscopy study of Ta2O5 based EIOS pH sensors in acid environment. Sensor Actuat B-Chem 192:399–405.https://doi.org/10.1016/j.snb.2013.10.129
Zhao R, Xu M, Wang J, Chen G (2010) A pH sensor based on the TiO2 nanotube array modified Ti electrode. Electrochim Acta 55:5647–5651. https://doi.org/10.1016/j.electacta.2010.04.102
Chou JC, Liao LP (2004) Study of TiO2 thin films for ion sensitive field effect transistor application with RF sputtering deposition. Jpn J Appl Phys 43:61–65. https://doi.org/10.1143/JJAP.43.61
Simić M, Manjakkal L, Zaraska K, Stojanović GM, Dahiya R (2017) TiO2 based thick film pH sensor. IEEE Sens J 17:248–255. https://doi.org/10.1109/JSEN.2016.2628765
Vasilyeva MS, Rudnev VS, Arefieva OD, Lapina AS, Plyusnina VI, Marinina GI (2016) Ti/TiO2 indicator electrodes formed by plasma electrolytic oxidation for potentiometric analysis. J Environ Anal Chem 96:1128–1144. https://doi.org/10.1080/03067319.2016.1243241
Kuo CY, Wang SJ, Ko RM, Tseng HH (2018) Super-Nernstian pH sensors based on WO3 nanosheets. Jpn J Appl Phys 57:04FM09. https://doi.org/10.7567/JJAP.57.04FM09
Santos L, Neto JP, Crespo A, Nunes D, Costa N, Fonseca IM, Barquinha P, Pereira L, Silva J, Martins R, Fortunato E (2014) WO3 nanoparticle-based conformable pH sensor. ACS Appl Mater Interf 6:12226–12234. https://doi.org/10.1021/am501724h
Teixeira MFS, Ramos LA, Fatibello-Filho O, Cavalheiro ETG (2001) PbO2-based graphite–epoxy electrode for potentiometric determination of acids and bases in aqueous and aqueous–ethanolic media. Fresenius J Anal Chem 370:383–386. https://doi.org/10.1007/s002160100728
Martinez-Manez R, Soto J, Garcia-Breijo E, Gil L, Ibanez J, Gadea E (2005) A multisensor in thick-film technology for water quality control. Sens Actuator A-Phys 120:589–595. https://doi.org/10.1016/j.sna.2005.03.006
Huang WD, Cao H, Deb S, Chiao M, Chiao JC (2011) A flexible pH sensor based on the iridium oxide sensing film. Sens Actuators A-Phys 169:1–11. https://doi.org/10.1016/j.sna.2011.05.016
Lonsdale W, Wajrak M, Alameh K (2018) Manufacture and application of RuO2 solid-state metal-oxide pH sensor to common beverages. Talanta 180:277–281. https://doi.org/10.1016/j.talanta.2017.12.070
Zhuiykov S (2009) Morphology of Pt-doped nanofabricated RuO2 sensing electrodes and their properties in water quality monitoring sensors. Sens Actuators B-Chem 136:248–256. https://doi.org/10.1016/j.snb.2008.10.030
Manjakkal L, Vilouras A, Dahiya R (2018) Screen printed thick film reference electrodes for electrochemical sensing. IEEE Sens J 18:7779–7785. https://doi.org/10.1109/JSEN.2018.2840349
Horton BE, Schweitzer S, DeRouin AJ, Ong KG (2011) A varactor-based, inductively coupled wireless pH sensor. IEEE Sens J 11:1061–1066. https://doi.org/10.1109/jsen.2010.2062503
Vasilyeva MS, Rudnev VS, Zabudskaya NE, Ustinov AY, Zasukhina LA, Marinina GI (2020) Preparation and study of Ti/TiO2, SbOx pH electrodes. J Anal Chem 75:246–253. https://doi.org/10.1134/S1061934820020173
Vasilyeva MS, Lukiyanchuk IV, Ustinov AY, Shchitovskaya EV, Marinina GI (2020) Anodic-cathodic formation of pH-sensitive TiO2-MoOx films on titanium. J Electroanal Chem 873:114388. https://doi.org/10.1016/j.jelechem.2020.114388
Lvov SN, Zhou XY, Ulmer GC, Barnes HL, Macdonald DD, Ulyanov SM, Benning LG, Grandstaff DE, Manna M, Vicenzi E (2003) Progress on yttria-stabilized zirconia sensors for hydrothermal pH measurements. Chem Geol 198:141–162. https://doi.org/10.1016/s0009-2541(03)00033-0
Light TS, Fletcher KS (1985) Evaluation of the zirconia pH sensor at 95° C. Anal Chim Acta 175:117–126. https://doi.org/10.1016/s0003-2670(00)82723-3
Niedrach LW (1980) A new membrane-type pH sensor for use in high temperature-high pressure water. J Electrochem Soc 127:2122–2130. https://doi.org/10.1149/1.2129358
Tsuruta T, Macdonald DD (1982) Stabilized ceramic membrane electrodes for the measurement of pH at elevated temperatures. J Electrochem Soc 129:1202–1210. https://doi.org/10.1149/1.2124090
Hettiarachchi S, Macdonald DD (1984) Ceramic membranes for precise pH measurements in high temperature aqueous environments. J Electrochem Soc 131:2206–2207. https://doi.org/10.1149/1.2116053
Danielson MJ, Koski OH, Meyers J (1985) Recent developments with high temperature stabilized-zirconia pH sensors. J Electrochem Soc 132:296–301. https://doi.org/10.1149/1.2113823
Seneviratne DS, Papangelakis VG, Zhou XY, Lvov SN (2003) Potentiometric pH measurements in acidic sulfate solutions at 250 °C relevant to pressure leaching. Hydrometallurgy 68:131–139. https://doi.org/10.1016/s0304-386x(02)00198-6
Betelu S, Polychronopoulou K, Rebholz C, Ignatiadis I (2011) Novel CeO2-based screen-printed potentiometric electrodes for pH monitoring. Talanta 87:126–135. https://doi.org/10.1016/j.talanta.2011.09.051
Aliofkhazraei M, Macdonald DD, Matykina E, Parfenov EV, Egorkin VS, Curran JA, Troughton SC, Sinebryukhov SL, Gnedenkov SV, Lampke T, Simchen F, Nabavi HF (2021) Review of plasma electrolytic oxidation of titanium substrates: mechanism, properties, applications, and limitations. Appl Surf Sci Adv 5:100121. https://doi.org/10.1016/j.apsadv.2021.100121
Kaseem M, Fatimah S, Nashrah N, Ko YG (2021) Recent progress in surface modification of metals coated by plasma electrolytic oxidation: principle, structure, and performance. Prog Mater Sci 117:100735. https://doi.org/10.1016/j.pmatsci.2020.100735
Simchen F, Sieber M, Kopp A, Lampke T (2020) Introduction to plasma electrolytic oxidation – an overview of the process and applications. Coatings 10:628. https://doi.org/10.3390/coatings10070628
Shin KR, Ko YG, Shin DH (2012) Surface characteristics of ZrO2-containing oxide layer in titanium by plasma electrolytic oxidation in K4P2O7 electrolyte. J Alloys Compd 536:S226–S230. https://doi.org/10.1016/j.jallcom.2011.11.037
Lederer S, Arat S, Fuerbeth W (2021) Influence of process parameters on the tribological behavior of PEO coatings on cp-titanium 4+ alloys for biomedical applications. Materials 14:5364. https://doi.org/10.3390/ma14185364
Molaei M, Nouri M, Babaei K, Fattah-Alhosseini A (2021) Improving surface features of PEO coatings on titanium and titanium alloys with zirconia particles: A review. Surf Interf 22:100888. https://doi.org/10.1016/j.surfin.2020.100888
Zhou K, Xie FQ, Wu XQ, Wang SQ (2020) Fabrication of high temperature oxidation resistance nanocomposite coatings on PEO treated TC21 alloy. Materials 13:11. https://doi.org/10.3390/ma13010011
Aliofkhazraei M, Gharabagh RS, Teimouri M, Ahmadzadeh M, Darband GB, Hasannejad H (2016) Ceria embedded nanocomposite coating fabricated by plasma electrolytic oxidation on titanium. J Alloys Compd 685:376–383. https://doi.org/10.1016/j.jallcom.2016.05.315
Di SC, Guo YP, Lv HW, Yu J, Li ZW (2015) Microstructure and properties of rare earth CeO2-doped TiO2 nanostructured composite coatings through micro-arc oxidation. Ceram Int 41:6178–6186. https://doi.org/10.1016/j.ceramint.2014.12.134
Yarovaya TP, Gordienko PS, Rudnev VS, Nedozorov PM, Zavidnaya AG (1994) Thin films containing transition element oxides – electrochemical synthesis at valve metal-surfaces. Russ J Electrochem 30:1276–1277
Yao ZP, Jiang YL, Jiang ZH, Wang FP, Wu ZD (2008) Preparation and structure of ceramic coatings containing zirconium oxide on Ti alloy by plasma electrolytic oxidation. J Mater Process Technol 205:303–307. https://doi.org/10.1016/j.jmatprotec.2007.11.112
Yao ZP, Su PB, Shen QX, Ju PF, Wu C, Zhai YF, Jiang ZH (2015) Preparation of thermal control coatings on Ti alloy by plasma electrolytic oxidation in K2ZrF6 solution. Surf Coat Technol 269:273–278. https://doi.org/10.1016/j.surfcoat.2015.01.032
Gao GR, Li Y, Hu D, Xi ZP (2018) Structure and infrared emissivity properties of the MAO coatings formed on TC4 alloys in K2ZrF6-based solution. Materials 11:254. https://doi.org/10.3390/ma11020254
Yang C, Cui SH, Wu ZC, Zhu JY, Huang J, Ma ZY, Fu RKY, Tian XB, Chu PK, Wu ZZ (2021) High efficient co-doping in plasma electrolytic oxidation to obtain long-term self-lubrication on Ti6Al4V. Tribol Int 160:107018. https://doi.org/10.1016/j.triboint.2021.107018
Rudnev VS, Kilin KN, Yarovaya TP, Nedozorov PM (2008) Oxide zirconium containing films on titanium. Protect Met 44:62–64. https://doi.org/10.1007/s11124-008-1008-8
Malyshev IV, Rudnev VS (2020) On the stability of ZrO2+TiO2 coatings on titanium formed by plasma electrolytic oxidation in a chlorine-containing medium. Protect Met Phys Chem Surf 56:369–373. https://doi.org/10.1134/S2070205120020161
Rudnev VS, Malyshev IV, Lukiyanchuk IV, Kuryavyi VG (2012) Composition, surface structure, and thermal behavior of ZrO2 + TiO2/Ti and ZrO2 + CeOx + TiO2/Ti composites formed by plasma-electrolytic oxidation. Protect Met Phys Chem Surf 48:455–461. https://doi.org/10.1134/S207020511203015X
Rudnev VS, Yarovaya TP, Nedozorov PM, Ustinov AY, Tyrina LM, Malyshev IV, Kuryavyi VG, Egorkin VS, Sinebryukhov SL, Gnedenkov SV (2011) Obtaining ZrO2+CeOx+TiO2/Ti compositions by plasma-electrolytic oxidation of titanium and investigating their properties. Protect Met Phys Chem Surf 47:621–628. https://doi.org/10.1134/S2070205111050145
Tarkhanova IG, Bryzhin AA, Gantman MG, Yarovaya TP, Lukiyanchuk IV, Nedozorov PM, Rudnev VS (2019) Ce-, Zr-containing oxide layers formed by plasma electrolytic oxidation on titanium as catalysts for oxidative desulfurization. Surf Coat Technol 362:132–140. https://doi.org/10.1016/j.surfcoat.2019.01.101
Liu XY, Wang K, Zhou Y, Tang XY, Zhu XH, Zhang RS, Zhang XL, Jiang X, Liu BD (2019) In-situ fabrication of noble metal modified (Ce, Zr)O2-delta monolithic catalysts for CO oxidation. Appl Surf Sci 483:721–729. https://doi.org/10.1016/j.apsusc.2019.03.315
Shtefan VV, Smirnova AY (2015) Synthesis of Ce-, Zr-, and Cu-containing oxide coatings on titanium using microarc oxidation. Russ J Electrochem 51:1168–1175. https://doi.org/10.1134/S1023193515120101
Liu XY, Wang K, Zhou Y, Zhang XL, Tang XY, Ren PC, Jiang X, Liu BD (2019) In-situ fabrication of Ce-rich CeO2 nanocatalyst for efficient CO oxidation. J Alloys Compd 792:644–651. https://doi.org/10.1016/j.jallcom.2019.04.057
Zhang BP, Li B, Gao ST, Li YT, Cao R, Cheng JY, Li RP, Wang ER, Guo YM, Zhang KL, Liang J, Liu B (2020) Y-doped TiO2 coating with superior bioactivity and antibacterial property prepared via plasma electrolytic oxidation. Mater Des 192:108758. https://doi.org/10.1016/j.matdes.2020.108758
Lu XP, Mohedano M, Blawert C, Matykina E, Arrabal R, Kainer KU, Zheludkevich ML (2016) Plasma electrolytic oxidation coatings with particle additions – a review. Surf Coat Technol 307:1165–1182. https://doi.org/10.1016/j.surfcoat.2016.08.055
Wang C, Hao JM, Xing YZ, Guo CF, Chen H (2015) High temperature oxidation behavior of TiO2 + ZrO2 composite ceramic coatings prepared by microarc oxidation on Ti6Al4V alloy. Surf Coat Technol 261:201–207. https://doi.org/10.1016/j.surfcoat.2014.11.031
Umezawa Y, Buhlmann P, Umezawa K, Tohda K, Amemiya S (2000) Potentiometric selectivity coefficients of ion-selective electrodes part I. Inorganic cations (technical report). Pure Appl Chem 72:1851–2082. https://doi.org/10.1351/pac200072101851
Zeng YQ, Song W, Wang YA, Meng YH, Song FJ, Zhang SL, Zhong Q (2019) The utilization of dye wastewater in enhancing catalytic activity of CeO2-TiO2 mixed oxide catalyst for NO reduction and dichloromethane oxidation. Chemosphere 235:1146–1153. https://doi.org/10.1016/j.chemosphere.2019.07.031
Apelfeld AV, Ashmarin AA, Borisov AM, Vinogradov AV, Savushkina SV, Shmytkova EA (2017) Formation of zirconia tetragonal phase by plasma electrolytic oxidation of zirconium alloy in electrolyte comprising additives of yttria nanopowder. Surf Coat Technol 328:513–517. https://doi.org/10.1016/j.surfcoat.2016.09.071
Savushkina SV, Ashmarin AA, Apelfeld AV, Borisov AM, Vinogradov AV, Polyansky MN, Bogdashkina NL (2017) Investigation of zirconia tetragonal phase coatings formed by plasma electrolytic oxidation. J Phys: Conf Ser 857:012037. https://doi.org/10.1088/1742-6596/857/1/012037
Rudnev VS, Vaganov-Vil’kins AA, Ustinov AY, Nedozorov PM (2011) Carbon in oxide layers formed under electric discharge conditions. Prot Met Phys Chem Surf 47:330–338. https://doi.org/10.1134/C2070205111030130
Vovna VI, Gnedenkov SV, Gordienko PS, Kuznetsov MV, Sinebryukhov SL, Cherednichenko AI, Khrisanfova OA (1998) Surface layers produced on titanium by the microarc oxidation: an X-ray diffractometry study. Russ J Electrochem 34:1090–1093. WOS:000076740800021
French RH, Glass SJ, Ohuchi FS, Xu Y-N, Ching WY (1994) Experimental and theoretical determination of the electronic structure and optical properties of 3 phases of ZrO2. Phys Rev B 49:5134–5142. https://doi.org/10.1103/PhysRevB.49.5133
Zhang Y, Chen HX, Duan L, Fan JB (2021) The electronic structures, elastic constants, dielectric permittivity, phonon spectra, thermal properties and optical response of monolayer zirconium dioxide: a first-principles study. Thin Solid Films 721:138549. https://doi.org/10.1016/j.tsf.2021.138549
Polliotto V, Albanese E, Livraghi S, Indyka P, Sojka Z, Pacchioni G, Giamello E (2017) Fifty-fifty Zr-Ti solid solution with a TiO2-type structure: electronic structure and photochemical properties of zirconium titanate ZrTiO4. J Phys Chem C 121:5487–5497. https://doi.org/10.1021/acs.jpcc.6b12892
George A, Solomon S, Thomas JK, John A (2012) Characterizations and electrical properties of ZrTiO4 ceramic. Mater Res Bull 47:3141–3147. https://doi.org/10.1016/j.materresbull.2012.08.018
Lu X, Liang K, Gu S, Zheng Y, Fang H (1997) Effect of oxygen vacancies on transformation of zirconia at low temperatures. J Mater Sci 32:6653–6656. https://doi.org/10.1023/A:1018604504171
Safonov AA, Bagatur’yants AA, Korkin AA (2003) Oxygen vacancies in tetragonal ZrO2: ab initio embedded cluster calculations. Microelectronic Eng 69:629–632. https://doi.org/10.1016/S0167-9317(03)00355-1
Yashima M, Tsunekawa S (2006) Structures and the oxygen deficiency of tetragonal and monoclinic zirconium oxide nanoparticles. Acta Cryst B62:161–164. https://doi.org/10.1107/S0108768105030570
Polliotto V, Albanese E, Livraghi S, Agnoli S, Pacchioni G, Giamello E (2020) Structural, electronic and photochemical properties of cerium-doped zirconium titanate. Catal Today 340:49–57. https://doi.org/10.1016/j.cattod.2018.09.026
Iwahara H (1995) Technological challenges in the application of proton conducting ceramics. Solid State Ionics 77:289–298. https://doi.org/10.1016/0167-2738(95)00051-7
Iwahara H (1996) Proton conducting ceramics and their applications. Solid State Ionics 86–88:9–15. https://doi.org/10.1016/0167-2738(96)00087-2
Rudnev VS, Tyrina LM, Lukiyanchuk IV, Yarovaya TP, Malyshev IV, Ustinov AY, Nedozorov PM, Kaidalova TA (2011) Titanium-supported Ce-, Zr-containing oxide coatings modified by platinum or nickel and copper oxides and their catalytic activity in CO oxidation. Surf Coat Technol 206:417–424. https://doi.org/10.1016/j.surfcoat.2011.07.041
Sasikumar K, Bharathikannan R, Raja M, Mohanbabu B (2020) Fabrication and characterization of rare earth (Ce, Gd, and Y) doped ZrO2 based metal-insulator-semiconductor (MIS) type Schottky barrier diodes. Superlattices Microstruct 139:106424. https://doi.org/10.1016/j.spmi.2020.106424
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Crystallogr B. Struct Sci Cryst Eng Mater 25:925–946. https://doi.org/10.1107/S0567740869003220
Krogstad JA, Lepple M, Gao Y, Lipkin DM, Levi CG (2011) Effect of yttria content on the zirconia unit cell parameters. J Am Ceram Soc 94:4548–4555. https://doi.org/10.1111/j.1551-2916.2011.04862.x
Acknowledgements
The obtaining of PEO layers and their study by XRD and EDX methods were performed within the framework of the Institute of Chemistry FEB RAS State Order (project no. FWFN(0205)-2022-0001). Electroanalytical properties of the composites were studied at the Far Eastern Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vasilyeva, M.S., Lukiyanchuk, I.V., Yarovaya, T.P. et al. Plasma electrolytic synthesis and characterization of pH-sensitive TiO2-ZrO2-ZrTiO4-CeOx films on titanium. J Solid State Electrochem 27, 85–101 (2023). https://doi.org/10.1007/s10008-022-05306-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05306-8