Abstract
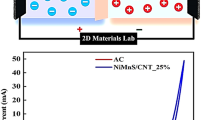
A hybrid supercapacitor, also known as a supercapattery, combines the high power density of supercapacitors with the high energy density of batteries. In this experiment, we used the hydrothermal technique to synthesize zinc sulfide (ZnS), strontium sulfide (SrS), and zinc strontium sulfide (ZnSrS). The density functional theory (DFT) revealed the metallic behavior of ZnSrS. The surface area measured through Brunauer–Emmett–Teller (BET) graphs for ZnSrS was 13.24 m2/g. The composite zinc strontium sulfide (ZnSrS) had a specific capacity of 469 C/g in three cell arrangement. An asymmetric supercapacitor was constructed using battery-graded zinc strontium sulfide (Zn50Sr50S) as the positive terminal and polyaniline doped activated carbon (PANI@AC) as the negative terminal. The supercapattery device (ZnSrS//PANI@AC) had a maximal capacity of 148 C/g and an energy density of 32.88 Wh/kg at the power density of 800 W/kg. After the completion of 5000 cycles, ZnSrS//PANI@AC retained 90% of its initial capacity. The exceptional electrochemical performance of ZnSrS demonstrates its application as a nanostructured electrode for future energy storage systems.










Similar content being viewed by others
References
Pant B, Ojha GP, Park M (2020) One-pot synthesis, characterization, and electrochemical studies of tin-nickel sulfide hybrid structures on nickel foam for supercapacitor applications. J Energ Stor 32:101954
Pant B, Park M, Ojha GP, Park J, Kuk Y-S, Lee E-J, Kim H-Y, Park S-J (2018) Carbon nanofibers wrapped with zinc oxide nano-flakes as promising electrode material for supercapacitors. J Colloid Interface Sci 522:40–47
Zhang LL, Zhao X (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38(9):2520–2531
Xu Y, Zhou Y, Guo J, Zhang S, Lu Y (2019) Preparation of SnS2/g-C3N4 composite as the electrode material for supercapacitor. J Alloy Compd 806:343–349
Raza W, Ali F, Raza N, Luo Y, Kim K-H, Yang J, Kumar S, Mehmood A, Kwon EE (2018) Recent advancements in supercapacitor technology. Nano Energy 52:441–473
Tasnin W, Saikia LC (2018) Performance comparison of several energy storage devices in deregulated AGC of a multi-area system incorporating geothermal power plant. IET Renew Power Gener 12(7):761–772
Zhu S, Chang Y, Hou W, Li Y, Ni J, Han G (2022) Molten-salt directed mesopore engineering of carbon nanotubes for energetic quasi-solid-state supercapacitors. Carbon 200:75–83
Zhu S, Wang Y, Zhang J, Sheng J, Yang F, Wang M, Ni J, Jiang H, Li Y (2022) Jahn‐Teller effect directed bandgap tuning of birnessite for pseudocapacitive application. Energ Environ Mater 12382
Hassan HU, Iqbal MW, Afzal AM, Abbas T, Zaka A, Yasmeen A, Noor NA, Aftab S, Ullah H (2022) Highly stable binary composite of nickel silver sulfide (NiAg2S) synthesized using the hydrothermal approach for high‐performance supercapattery applications. Int J Energ Res 46(8):11346–11358
Wu S, Zhu Y (2017) Highly densified carbon electrode materials towards practical supercapacitor devices. Sci China Mater 60(1):25–38
Zhang J, Zhao X (2012) On the configuration of supercapacitors for maximizing electrochemical performance. Chemsuschem 5(5):818–841
Dubal DP, Chodankar NR, Kim D-H, Gomez-Romero P (2018) Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem Soc Rev 47(6):2065–2129
Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442(7100):282–286
Chen F, Cao Y, Jia D (2013) A facile route for the synthesis of ZnS rods with excellent photocatalytic activity. Chem Eng J 234:223–231
Jayalakshmi M, Rao MM (2006) Synthesis of zinc sulphide nanoparticles by thiourea hydrolysis and their characterization for electrochemical capacitor applications. J Power Sources 157(1):624–629
Wang Y, Herron N (1987) Chemical effects on the optical properties of semiconductor particles. J Phys Chem 91(19):5005–5008
Søgaard M, Hendriksen PV, Mogensen M, Poulsen FW, Skou E (2006) Oxygen nonstoichiometry and transport properties of strontium substituted lanthanum cobaltite. Solid State Ionics 177(37–38):3285–3296
Aguadero A, Fawcett L, Taub S, Woolley R, Wu K-T, Xu N, Kilner JA, Skinner SJ (2012) Materials development for intermediate-temperature solid oxide electrochemical devices. J Mater Sci 47(9):3925–3948
Gao Z, Mogni LV, Miller EC, Railsback JG, Barnett SA (2016) A perspective on low-temperature solid oxide fuel cells. Energy Environ Sci 9(5):1602–1644
Iqbal MZ, Khan A, Numan A, Haider SS, Iqbal J (2019) Ultrasonication-assisted synthesis of novel strontium based mixed phase structures for supercapattery devices. Ultrason Sonochem 59:104736
Dell’Agli G, Spiridigliozzi L, Marocco A, Accardo G, Frattini D, Kwon Y, Yoon S (2017) Morphological and crystalline evolution of Sm-(20 mol%)–doped ceria nanopowders prepared by a combined co-precipitation/hydrothermal synthesis for solid oxide fuel cell applications. Ceram Int 43(15):12799–12808
Ramachandran R, Saranya M, Kollu P, Raghupathy BP, Jeong SK, Grace AN (2015) Solvothermal synthesis of Zinc sulfide decorated Graphene (ZnS/G) nanocomposites for novel Supercapacitor electrodes. Electrochim Acta 178:647–657
Iqbal MF, Ashiq MN, Razaq A, Saleem M, Parveen B, Hassan M-U (2018) Excellent electrochemical performance of graphene oxide based strontium sulfide nanorods for supercapacitor applications. Electrochim Acta 273:136–144
Dai S, Zhao B, Qu C, Chen D, Dang D, Song B, Deglee BM, Fu J, Hu C, Wong C-P (2017) Controlled synthesis of three-phase NixSy/rGO nanoflake electrodes for hybrid supercapacitors with high energy and power density. Nano Energy 33:522–531
Iqbal MF, Ashiq MN, Iqbal S, Bibi N, Parveen B (2017) High specific capacitance and energy density of synthesized graphene oxide based hierarchical Al2S3 nanorambutan for supercapacitor applications. Electrochim Acta 246:1097–1103
Niu C, Sichel EK, Hoch R, Moy D, Tennent H (1997) High power electrochemical capacitors based on carbon nanotube electrodes. Appl Phys Lett 70(11):1480–1482
Zou R, Xu K, Wang T, He G, Liu Q, Liu X, Zhang Z, Hu J (2013) Chain-like NiCo 2 O 4 nanowires with different exposed reactive planes for high-performance supercapacitors. J Mater Chem A 1(30):8560–8566
Brousse T, Bélanger D, Long JW (2015) To be or not to be pseudocapacitive? J Electrochem Soc 162(5):A5185
Li J, Zhao W, Huang F, Manivannan A, Wu N (2011) Single-crystalline Ni (OH) 2 and NiO nanoplatelet arrays as supercapacitor electrodes. Nanoscale 3(12):5103–5109
Sharma M, Sundriyal S, Panwar AK, Gaur A (2019) Facile synthesis and electrochemical performance of Mg-substituted Ni1-xMgxCo2O4 mesoporous nanoflakes for energy storage applications. Electrochim Acta 294:53–59
Qu Z, Shi M, Wu H, Liu Y, Jiang J, Yan C (2019) An efficient binder-free electrode with multiple carbonized channels wrapped by NiCo2O4 nanosheets for high-performance capacitive energy storage. J Power Sources 410:179–187
Ghadimi LS, Arsalani N, Tabrizi AG, Mohammadi A, Ahadzadeh I (2018) Novel nanocomposite of MnFe2O4 and nitrogen-doped carbon from polyaniline carbonization as electrode material for symmetric ultra-stable supercapacitor. Electrochim Acta 282:116–127
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl Mater Interfaces 5(6):2188–2196
Omar FS, Numan A, Duraisamy N, Ramly MM, Ramesh K, Ramesh S (2017) Binary composite of polyaniline/copper cobaltite for high performance asymmetric supercapacitor application. Electrochim Acta 227:41–48
Iqbal MZ, Faisal MM, Ali SR, Afzal AM, Karim MRA, Kamran MA, Alharbi T (2020) Strontium phosphide-polyaniline composites for high performance supercapattery devices. Ceram Int 46(8):10203–10214
Faisal MM, Ali SR, Iqbal MZ, Iqbal MW, Numan A, Sanal K (2021) Effect of polyaniline on the performance of zinc phosphate as a battery-grade material for supercapattery. J Energ Stor 44:103329
Iqbal MZ, Zakar S, Haider SS, Afzal AM, Iqbal MJ, Kamran MA, Numan A (2020) Electrodeposited CuMnS and CoMnS electrodes for high-performance asymmetric supercapacitor devices. Ceram Int 46(13):21343–21350
Duraisamy N, Numan A, Fatin SO, Ramesh K, Ramesh S (2016) Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J Colloid Interface Sci 471:136–144
Frackowiak E, Metenier K, Bertagna V, Beguin F (2000) Supercapacitor electrodes from multiwalled carbon nanotubes. Appl Phys Lett 77(15):2421–2423
Macdonald JR, Barsoukov E (2018) Impedance spectroscopy: theory, experiment, and applications, John Wiley & Sons
Iqbal MZ, Faisal MM, Ali SR, Farid S, Afzal AM (2020) Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim Acta 346:136039
Li Z, Zhou Z, Yun G, Shi K, Lv X, Yang B (2013) High-performance solid-state supercapacitors based on graphene-ZnO hybrid nanocomposites. Nanoscale Res Lett 8(1):1–9
Kale SB, Lokhande AC, Pujari RB, Lokhande CD (2018) Cobalt sulfide thin films for electrocatalytic oxygen evolution reaction and supercapacitor applications. J Colloid Interface Sci 532:491–499
Liang K, DeNaufville J, Jacobson A, Chianelli R, Betts F (1980) Structure of amorphous transition metal sulfides. J Non-Cryst Solids 35:1249–1254
Pujari SS, Kadam SA, Ma Y-R, Katkar PK, Marje SJ, Khalate SA, Lokhande AC, Patil UM (2020) Facile synthesis of microstrip-like copper phosphate hydroxide thin films for supercapacitor applications. J Electron Mater 49(6):3890–3901
Zhong Y, Cao X, Ying L, Cui L, Barrow C, Yang W, Liu J (2020) Homogeneous nickel metal-organic framework microspheres on reduced graphene oxide as novel electrode material for supercapacitors with outstanding performance. J Colloid Interface Sci 561:265–274
Datta J, Das M, Sil S, Kumar S, Dey A, Jana R, Bandyopadhyay S, Ray PP (2019) Improvement of charge transport for hydrothermally synthesized Cd0. 8Fe0. 2S over co-precipitation method: A comparative study of structural, optical and magnetic properties. Mater Sci Semiconduc Process 91:133–145
Acknowledgements
The Higher Education Commission (HEC) of Pakistan financed this research under the National Research Program for Universities (NRPU), project number HEC/R&D/NRPU/2017/7876. The authors would like to thank Riphah International University for sponsoring this research under the project number Riphah-ORIC-21-22/FEAS-04.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, H., Iqbal, M.W., Afzal, A.M. et al. Enhanced the performance of zinc strontium sulfide-based supercapattery device with the polyaniline doped activated carbon. J Solid State Electrochem 27, 125–137 (2023). https://doi.org/10.1007/s10008-022-05305-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05305-9