Abstract
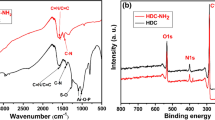
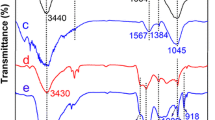
In this paper, polyphosphazene was synthesized as precursors using benzene-1,4-diboronic acid and hexachlorocyclotriphosphazene (HCCP) by a simple one-step ultrasonic method. Furthermore, B, N, and P co-doped carbon materials containing phosphate functional groups (denoted as BNPCs-PO4) was obtained by the carbonization and functionalization of precursors at high temperature. Through the structural characterization and the investigation of electrochemical and electrosorption properties, it can be proved that BNPCs-PO4 has excellent porous structure and electrosorption properties with the maximum electrosorption capacity of 750 ± 40 mg/g for U(VI) and the equilibrium time of 24 min. The optimal pH and applied potential for electrosorption were 5.0 and 0.9 V, respectively. Meanwhile, it also showed high selectivity under the coexistence of multiple metal ions and excellent recyclability after six cycles. The results showed the introduction of heteroatoms improved the electrosorption properties of carbon materials and BNPCs-PO4 can be utilized as electrode for the removal of U(VI) in aqueous solution.












Similar content being viewed by others
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
All code generated or used during the study are available from the corresponding author by request.
References
Hu BW, Ye F, Ren XM, Zhao DL, Sheng GD, Li H, Ma JY, Wang XK, Huang YY (2016) X-ray absorption fine structure study of enhanced sequestration of U(VI) and Se(IV) by montmorillonite decorated with zero-valent iron nanoparticles. Environ Sci Nano 3(6):1460–1472
Wang XL, Guo HX, Wang F, Tan TS, Zhang HX (2020) Halloysite nanotubes: an eco-friendly adsorbent for the adsorption of Th(IV)/U(VI) ions from aqueous solution. J Radioanal Nucl Chem 324(3):1151–1165
Zheng Z, Wang Y, Zhao W, Xiong G, Cao X, Dai Y, Le Z, Yu S, Zhang Z, Liu Y (2017) Adsorptive removal of uranyl ions in aqueous solution using hydrothermal carbon spheres functionalized with 4-aminoacetophenone oxime group. J Radioanal Nucl Chem 312(2):187–198
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134(39):16441–16446
Fourie M, Meyer WCMH, Van Der Westhuizen DJ, Krieg HM (2016) Uranium recovery from simulated molybdenum-99 production residue using non-dispersive membrane based solvent extraction. Hydrometallurgy 164:330–333
Vigier JF, Laplace A, Renard C, Miguirditchian M, Abraham F (2018) Uranium (III)-plutonium (III) co-precipitation in molten chloride. J Nucl Mater 499:394–400
Hoyer M, Zabelt D, Steudtner R, Brendler V, Haseneder R, Repke JU (2014) Influence of speciation during membrane treatment of uranium contaminated water. Sep Purif Technol 132:413–421
Biesheuvel PM, Porada S, Levi M, Bazant MZ (2014) Attractive forces in microporous carbon electrodes for capacitive deionization. J Solid State Electrochem 18:1365–1376
Zafra MC, Lavela P, Rasines G, Macías C, Tirado JL (2014) Effect of the resorcinol/catalyst ratio in the capacitive performance of carbon xerogels with potential use in sodium chloride removal from saline water. J Solid State Electrochem 18:2847–2856
Wei YC, Huo YQ, Tian GY, Meng QH, Cao B (2016) Nitrogen-doped functional graphene nanocomposites for capacitive deionization of NaCl aqueous solutions. J Solid State Electrochem 20:2351–2362
Wang SS, Feng JW, Meng QH, Cao B, Tian GY (2019) Study on boron and nitrogen co-doped graphene xerogel for high-performance electrosorption application. J Solid State Electrochem 23:2377–2390
Cen BQ, Li KX, Lv CC, Yang R (2020) A novel asymmetric activated carbon electrode doped with metal -organic frameworks for high desalination performance J Solid State. Electrochem 24:687–697
Sufiani O, Elisadiki J, Machunda RL, Jande YAC (2019) Modification strategies to enhance electrosorption performance of activated carbon electrodes for capacitive deionization applications. J Electroanal Chem 848:113328
Liu NL, Sun SH, Hou CH (2019) Studying the electrosorption performance of activated carbon electrodes in batch-mode and single-pass capacitive deionization. Sep Purif Technol 215:403–409
Feng JH, Xiong S, Wang Y (2019) Atomic layer deposition of TiO2 on carbon-nanotube membranes for enhanced capacitive deionization. Sep Purif Technol 213:70–77
Bao SX, Duan JH, Zhang YM (2018) Characteristics of nitric acid-modified carbon nanotubes and desalination performance in capacitive deionization. Chem Eng Technol 41(9):1793–1799
Qian M, Duan M, Gong Z (2019) Nitrogen doped self-shrinking porous 3D graphene capacitor deionization electrode. Int J Energ Res 43(13):7583–7593
Roberts AD, Li X, Zhang H (2014) Porous carbon spheres and monoliths: morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem Soc Rev 45(34):4341–4356
Liu W, Zhang SK, Dar SU, Zhao Y, Akram R, Zhang XF, Jin S, Wu ZP, Wu DZ (2018) Polyphosphazene-derived heteroatoms-doped carbon materials for supercapacitor electrodes. Carbon 129:420–427
Liu XP, Liu Y, Wang Y, Yuan DZ, Liu JB, Chew JW (2021) Preparation of porous carbon materials by polyphosphazene as precursor for sorption of U(VI). Colloid Interf Sci 41:100387
Zhao WH, Lin XY, Qin Y, Cai HM, Chen YL, Luo XG (2017) Preparation of chemically oxidized porous carbon and its adsorption of uranium(VI) from aqueous solution. J Radioanal Nucl Chem 314(3):1853–1864
Bayramoglu G, Arica MY (2016) Amidoxime functionalized Trametes trogii pellets for removal of uranium (VI) from aqueous medium. J Radioanal Nucl Chem 307(1):373–384
Wang YQ, Zhang ZB, Liu YH, Cao XH, Liu YT, Qin L (2012) Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem Eng J 198:246–253
Li L, Huang S, Wen T, Ma R, Yin L, Li J, Chen Z, Hayat T, Hu B, Wang X (2019) Fabrication of carboxyl and amino functionalized carbonaceous microspheres and their enhanced adsorption behaviors of U(VI). J Colloid Interf Sci 543:225–236
Sun G, Ma L, Ran J, Li B, Shen X, Tong H (2016) Templated synthesis and activation of highly nitrogen-doped worm-like carbon composites based on melamine-urea-formaldehyde resins for high performance supercapacitors. Electrochim Acta 194:168–178
Yu J, Guo M, Muhammad F, Wang A, Zhang F, Li Q, Zhu G (2014) One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol–urea–formaldehyde resin for CO2 capture. Carbon 69:502–514
Jiang J, Chen H, Wang Z, Bao L, Qiang Y, Guan S, Chen J (2015) Nitrogen-doped hierarchical porous carbon microsphere through KOH activation for supercapacitors. J Colloid Interf Sci 452:54–61
Bai C, Zhang M, Bo L, Yin T, Li S (2015) Three novel triazine-based materials with different O/S/N set of donor atoms: one-step preparation and comparison of their capability in selective separation of uranium. J Hazard Mater 300:368–377
Huang XB, Wang Q, Jiang D, Huang YM (2017) Facile synthesis of B. N co-doped three-dimensional porous graphitic carbon toward oxygen reduction reaction and oxygen evolution reaction, Catal Commun 100:89–92
Zeng K, Su JM, Cao XC, Zheng XJ, Li XW, Tian JH, Jin C, Yang RZ (2020) B, N co-doped ordered mesoporous carbon with enhanced electrocatalytic activity for the oxygen reduction reaction. J Alloys Compd 824:153908
Liao Y, Wang M, Chen DJ (2019) Electrosorption of uranium(VI) by highly porous phosphate-functionalized graphene hydrogel. Appl Surf Sci 484:83–96
Zhao S, Liu J, Li C, Ji W, Yang M, Huang H, Liu Y, Kang Z (2014) Tunable ternary (N, P, B)-doped porous nanocarbons and their catalytic properties for oxygen reduction reaction. ACS Appl Mater Inter 6(24):22297–22304
Liu W, Zhang SK, Dar SU, Zhao Y, Akram R, Zhang XF, Jin S, Wu ZP, Wu DZ (2017) Polyphosphazene-derived heteroatoms-doped carbon materials for supercapacitor electrodes. Carbon 129:420–427
Li Y, Liu Y, Shen J, Qi J, Li J, Sun X, Shen J, Han W, Wang L (2018) Design of nitrogen-doped cluster-like porous carbons with hierarchical hollow nanoarchitecture and their enhanced performance in capacitive deionization. Desalination 430:45–55
Yun L, Wang M, Chen DJ (2018) Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium(VI) adsorption. Ind Eng Chem Res 57:8472–8483
Wu ZS, Winter A, Chen L, Sun Y, Turchanin A, Feng XL, Müllen K (2012) Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv Mater 24(37):5130–5135
Xue Y, Cao M, Gao JZ, Gui YY, Chen JQ, Liu P, Ma FQ, Yan YD, Qiu M (2021) Electroadsorption of uranium on amidoxime modified graphite felt. Sep Purif Technol 255:117753
Wu XD, Li KW, Ying SJH, Liu L, Wang M, Liao Y (2019) Three-dimensional graphene materials for UO22+ electrosorption. J Radioanal Nucl Chem 321(3):977–984
Zhou J, Zhou HJ, Zhang YZ, Wu J, Zhang HM, Wang GZ, Li JX (2020) Pseudocapacitive deionization of uranium(VI) with WO3/C electrode. Chem Eng J 398:125460
Jung CH, Lee HY, Moon JK, Won HJ, Shul YG (2010) Electrosorption of uranium ions on activated carbon fibers. J Radioanal Nucl Chem 287(3):833–839
Funding
This work was financially supported by the National Natural Science Foundation of China (21966005, 22066002), the Natural Science Foundation of Jiangxi Province (20202BABL203001, 20192BAB202007, 20192ACB21001), the Opening fund project of State Key Laboratory of Nuclear Resources and Environment, East China University of Technology (NRE1926).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Liu, Y., Wang, Y. et al. Efficient electrosorption of uranium(VI) by B, N, and P co-doped porous carbon materials containing phosphate functional groups. J Solid State Electrochem 25, 2443–2454 (2021). https://doi.org/10.1007/s10008-021-05029-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-05029-2