Abstract
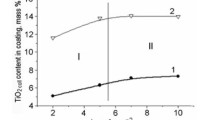
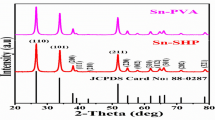
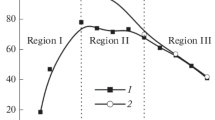
The electrodeposition of PbO2 from sodium laureth sulfate-containing medium has been investigated. It has been established that at low anodic polarizations, second electron transfer step is rate determining, whereas at high anodic polarizations, such step is diffusion transport of lead ions to the electrode surface. The presence of sodium laureth sulfate in the deposition electrolyte leads to a slight inhibition of the deposition of lead dioxide. It has been found that the morphology and structure of composite materials differs significantly from lead dioxide. With an increase in the additive content in the composite, there is a transition from large-grained deposits to materials with submicron and nano-sized crystals. It is shown that anionic surfactants, sodium laureth sulfate in particular, are included in the growing lead dioxide. The content of organic substance in the oxide can vary from 3.2 to 12.5 wt.%, forming a surfactant-oxide composite coating. The electrocatalytic activity of the materials involved was investigated with respect to oxygen evolution reaction and the oxidation of 4-chlorophenol. The calculated value of Tafel slope is 229 on non-modified PbO2, while on 10.2 wt.% sodium laureth sulfate-PbO2, it is 178 mV/dec. According to absorption spectra, the initial solution of chlorophenol is characterized by two peaks at 220 and 280 nm. At first, the electrolysis shows a decrease in the peak at 220 nm, as well as a slight increase in the peak at 280 nm and the appearance of the plateau at 240–270 nm, which is caused by a decrease in the concentration of 4-chlorophenol and accumulation of benzoquinone in the solution. A further increase in the time of electrolysis leads to the disappearance of the peaks at 220 and 280 nm, as well as the reduction of the plateau at 240–270 nm due to a decrease in the concentrations of both 4-chlorophenol and benzoquinone. Already after 4 h of electrolysis, the aromatic compounds are completely destroyed with the formation of only aliphatic electrolysis products (mainly maleic acid), which was evidenced by high performance liquid chromatography. The processes of electrooxidation of 4-chlorophenol on lead dioxide and PbO2-based composite materials proceed qualitatively in the same way and differ only in the rate.











Similar content being viewed by others
References
Bi Q, Guan W, Gao Y, Cui Y, Ma S, Xue J (2019) Study of the mechanisms underlying the effects of composite intermediate layers on the performance of Ti/SnO 2 -Sb-La electrodes. Electrochim Acta 306:667–679
Du H, Duan G, Vang N, Liu J, Tang Y, Pang R, Chen Y, Wan P (2018) Fabrication of Ga2 O3 –PbO2 electrode and its performance in electrochemical advanced oxidation processes. J Solid State Electrochem 22(12):3799–3806
Pereyra J, Martinez MV, Barbero C, Bruno M, Acevedo D (2019) Hydrogel-graphene oxide nanocomposites as electrochemical platform to simultaneously determine dopamine in presence of ascorbic acid using an unmodified glassy carbon electrode. J Compos Sci 3:1–14
Vargas R, Borras C, Mendez D, Mostany J, Scharifker BR (2016) Electrochemical oxygen transfer reactions: electrode materials, surface processes, kinetic models, linear free energy correlations, and perspectives. A review. J Solid State Electrochem 20:875–893
Santos JEL, de Moura DC, da Silva DR, Panizza M, Martinez-Huitle CA (2019) Application of TiO2-nanotubes/PbO2 as an anode for the electrochemical elimination of acid red 1 dye. J Solid State Electrochem 23:351–360
Labbe F, Asset T, Chatenet M, Ahmad Y, Guerin K, Metkemeijer R, Berthon-Fabry S (2019) Activity and durability of platinum-based electrocatalysts with tin oxide–coated carbon aerogel materials as catalyst supports. Electrocatalysis 10:156–172
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828
Berenguer R, Quijada C, La Rosa-Toro A, Morallon E (2019) Electro-oxidation of cyanide on active and non-active anodes: designing the electrocatalytic response of cobalt spinels. Sep Purif Technol 208:42–50
Pouladvand I, Asl SK, Hoseini MG, Rezvani M (2019) Characterization and electrochemical behavior of Ti/TiO2–RuO2–IrO2–SnO2 anodes prepared by sol–gel process. J Sol-Gel Sci Technol 89:553–561
Velichenko AB, Knysh VA, Luk’yanenko TV, Velichenko YA, Devilliers D (2012) Electrodeposition PbO2-TiO2 and PbO2-ZrO2 and its physicochemical properties. Mater Chem Phys 131:686–693
Li X, Xu H, Yan W (2017) Effects of twelve sodium dodecyl sulfate (SDS) on electro-catalytic performance and stability of PbO2electrode. J Alloys Compd 718:386–395
Shmychkova O, Luk'yanenko T, Amadelli R, Velichenko A (2016) Electrodeposition of Ni2+-doped PbO2 and physicochemical properties of the coating. J Electroanal Chem 774:88–94
Boyes W (2010) Instrumentation reference book, 4th edn. Elsevier, Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo
Li D (2008) Encyclopedia of microfluidics and nanofluidics. Springer Boston MA
Robinson JW, Frame EMS, Frame GM II (2005) Undergraduate instrumental analysis, 6th edn. Marcel Dekker, New York
Adamson AW, Gast AP (1997) Physical chemistry of surfaces, 6th edn Wiley Interscience
Velichenko AB, Luk'yanenko TV, Nikolenko NV, Amadelli R, Danilov FI (2007) Nafion effect on the lead dioxide electrodeposition kinetics. Russ J Electrochem 43:118–120
Nikolenko NV, Esajenko EE (2005) Surface properties of synthetic calcium hydroxyapatite. Adsorpt Sci Technol 23:543–553
Campbell SA, Peter LM (1991) Detection of soluble intermediates during deposition and reduction of lead dioxide. J Electroanal Chem 306:185–194
Shmychkova O, Luk'yanenko T, Velichenko A, Meda L, Amadelli R (2013) Bi-doped PbO2 anodes: electrodeposition and physico-chemical properties. Electrochim Acta 111:332–338
Li X, Pletcher D, Walsh FC (2011) Electrodeposited lead dioxide coatings. Chem Soc Rev 40:3879–3894
Fleischmann M, Liler M (1958) The anodic oxidation of solutions of plumbous salts. Part 1. The kinetics of deposition of α-lead dioxide from acetate solutions. Trans Faraday Soc 54:1370–1381
Fleischmann M, Thirsk HR (1959) The potentiostatic study of the growth of deposits on electrodes. Electrochim Acta 1:146–160
Fleischmann M, Mansfield JR, Thirsk HR, Wilson HGE, Wynne-Jones L (1967) The investigation of the kinetics of electrode reactions by the application of repetitive square pulses of potential. Electrochim Acta 12:967–982
The electrochemistry of lead / [ed. AT Kuhn]. – New York: Academic Press, 1979
Abyaneh MY, Fleischmann M, Del Giudice E (2009) The investigation of nucleation using microelectrodes: I. the ensemble averages of the times of birth of the first nucleus. Electrochim Acta 54:879–887
Abyaneh MY, Saez V, Gonzalez-Garcia J, Mason TJ (2010) Electrocrystallization of lead dioxide: analysis of the early stages of nucleation and growth. Electrochim Acta 55:3572–3579
Chang H, Johnson DC (1989) Electrocatalysis of anodic oxygen-transfer reaction. Chronoamperometric and voltammetric studies of the nucleation and electrodeposition of β-lead dioxide at a rotated gold disk electrode in acidic media. J Electrochem Soc 136:17–22
Velicheko AB, Girenko DV, Danilov FI (1995) Electrodeposition of lead dioxide at an Au electrode. Electrochim Acta 40:2803–2807
Velicheko AB, Girenko DV, Danilov FI (1996) Mechanism of lead dioxide electrodeposition. J Electroanal Chem 425(1):127–132
Velichenko AB, Baranova EA, Girenko DV, Amadelli R, Kovalyov SV, Danilov FI (2003) Mechanism of electrodeposition of lead dioxide from nitrate solutions. Russ J Electrochem 39:615–621
Shmychkova O, Luk'yanenko T, Amadelli R, Velichenko A (2014) Physico-chemical properties of PbO2-anodes doped with Sn4+and complex ions. J Electroanal Chem 717-718:196–201
Knysh V, Luk’yanenko T, Shmychkova O, Amadelli R, Velichenko A (2017) Electrodeposition of composite PbO2-TiO2 materials from colloidal methanesulfonate electrolytes. J Solid State Electrochem 21:537–544
Saez V, Marchante E, Diez MI, Esclapez MD, Bonete P, Lana-Villarreal T, Gonzalez Garcia J, Mostany J (2011) A study of the lead dioxide electrocrystallization mechanism on glassy carbon electrodes. Part I: experimental conditions for kinetic control. Mater Chem Phys 125:46–54
Abyaneh MY (2014) Electrocrystallization: modeling and its application, in Developments in electrochemistry: science inspired by Martin Fleischmann (eds D Pletcher, Z-Q Tian and DE Williams), John Wiley & Sons, Ltd, Chichester, UK https://doi.org/10.1002/9781118694404.ch3
Gonzalez-Garcia J, Gallud F, Iniesta J, Montiel V, Aldaz A, Lasia A (2000) Kinetics of electrocrystallization of PbO2 on glassy carbon electrodes. Partial inhibition of the progressive three-dimensional nucleation and growth. J Electrochem Soc 147:2969–2974
Gonzalez-Garcia J, Gallud F, Iniesta J, Montiel V, Aldaz A, Lasia A (2001) Kinetics of electrocrystallization of PbO2 on glassy carbon electrodes. Influence of the electrode rotation. Electroanalysis 13:1258–1264
Luk’yanenko T, Velichenko A, Knysh V, Shmychkova O (2018) Influence of methanesulfonate ions on electrooxidation of Pb(II). Voprosy Khimii i Khimicheskoi Tekhnologii 4:27–35
Shmychkova O, Luk'yanenko T, Piletska A, Velichenko A, Gladyshevskii R, Demchenko P, Amadelli R (2015) Electrocrystallization of lead dioxide: influence of early stages of nucleation on phase composition. J Electroanal Chem 746:57–61
Nunez M (2007) Trends in electrochemistry research. Nova Publ Inc, N-Y
Ghasemi S, Mousavi MF, Shamsipur M (2007) Electrochemical deposition of lead dioxide in the presence of polyvinylpyrrolidone. A morphological study. Electrochim Acta 53:459–467
Ruetschi P, Giovanoli R (1991) On the presence of OH− ions, Pb2+ ions and cation vacancies in PbO2. Power Sources 13:81–97
Pavlov D, Monahov B, Petrov D (2000) Influence of Ag as alloy additive on the oxygen evolution reaction on Pb/PbO2 electrode. J Power Sources 85:59–62
Shmychkova O, Luk’yanenko T, Dmitrikova L, Velichenko A (2019) Modified lead dioxide for organic wastewater treatment: physico-chemical properties and electrocatalytic activity. J Serb Chem Soc 84:187–198
Trasatti S, Lodi G (1981) Electrodes of conductive metallic oxides. Part B, Elsevier, Amsterdam, Holland
Shmychkova O, Luk'yanenko T, Yakubenko A, Amadelli R, Velichenko A (2015) Electrooxidation of some phenolic compounds at Bi-doped PbO2. Appl Catal B 162:346–351
Amadelli R, Maldotti A, Molinari A, Danilov FI, Velichenko AB (2002) Influence of the electrode history and effects of the electrolyte composition and temperature on O2 evolution at β-PbO2-anodes in acid media. J Electroanal Chem 534:1–12
Shmychkova O, Luk’yanenko T, Dmitrikova L, Velichenko A (2018) Electrooxidation of 4-clorphenol on modified lead dioxide anodes. Voprosy Khimii i Khimicheskoi Tekhnologii 3:50–57
Acknowledgments
The authors are indebted to Rossano Amadelli (Universita’ degli Studi di Ferrara) for help in the discussion of obtained results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luk’yanenko, T., Shmychkova, O. & Velichenko, A. PbO2-surfactant composites: electrosynthesis and catalytic activity. J Solid State Electrochem 24, 1045–1056 (2020). https://doi.org/10.1007/s10008-020-04572-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04572-8