Abstract
The charge-discharge characteristics and the aging mechanism of PbO2 layers doped with bismuth in contact with sulfuric acid solutions were studied by using combined cyclic voltammetry and electrochemical quartz crystal microbalance (EQCM) techniques. For this purpose, thick lead dioxide layers (non-doped and doped with Bi) were electrodeposited on gold substrate from aqueous solutions of Pb(NO3)2 dissolved in nitric acid and they were investigated in sulfuric acid media. Based on the electrochemical and the mass change responses, it is concluded that during the electrodeposition, bismuth influences the structure of the PbO2 formed. Bi(III) also inhibits the oxidation of lead sulfate and affects the reduction kinetics of lead dioxide. During successive cyclization (aging), the presence of bismuth accelerates the hydration of PbO2.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Porous lead dioxide materials are used in lead-acid batteries [1,2,3,4] as well as in many industrial applications such as wastewater treatment for oxidation of organic compounds [5,6,7,8,9,10,11,12,13]. The electrochemical behavior of pure lead dioxide layers (charge-discharge characteristics, aging processes) has been widely studied previously [14,15,16,17,18,19].
In order to improve the electrochemical and mechanical properties of PbO2 layers, several investigations have been carried out, e.g., by using different metals or metal oxides [20,21,22] including bismuth [23, 24]. However, the results and the explanations of some studies are confusing regarding to the effects of these additives on certain properties. In his review [25], Koop also stated that the published experimental evidences for both the perceived and actual effects of bismuth on lead-acid battery performance are complex and often contradictory. Therefore, it is evident that electrochemical studies combined with supplementary techniques are necessary to gain a deeper understanding of the complex behavior of PbO2 electrode doped with bismuth.
The electrochemical quartz crystal microbalance (EQCM) is a powerful technique for monitoring in situ surface mass changes during different physico-chemical processes. Based on the Sauerbrey equation [26] and Faraday’s law of electrolysis, effective molar mass changes related to the participating species can be calculated. Surprisingly, the EQCM technique has rarely been used for monitoring the changes occurring in the course of charging-discharging of PbO2 layers [19, 27, 28].
The main goal of this paper is to study the effects of bismuth additive on the charge-discharge characteristics of electrodeposited lead dioxide layer on gold substrate in sulfuric acid solution and its influence on the aging mechanism during cyclization.
Experimental
A conventional three-electrode electrochemical cell was used containing a gold-coated quartz crystal as the working electrode (5 MHz AT-cut, the geometric area during electrodeposition was 1.37 cm2) and a gold plate as the counter electrode. A spatially separated sodium chloride saturated calomel electrode (SCE) was used as the reference electrode. Lead(II) and Bi(III) solutions were prepared by dissolving Pb(NO3)2 and Bi(NO3)3, respectively, in ultrapure water (Millipore) and nitric acid (Merck). Sulfuric acid solutions saturated with bismuth sulfate were made by dissolving Bi(NO3)3 in sulfuric acid (Merck) until Bi2(SO4)3 was precipitated. The electrolytes were deaerated to remove the dissolved oxygen by bubbling high purity argon for 15 min.
The relationship between the measured frequency change (Δf) and the mass change per unit area (Δm) is given by the Sauerbrey equation [26]:
where Cf is the integral sensitivity factor for the crystal used which is 56.6 Hz μg−1 cm2 for a 5 MHz AT-cut quartz crystal at 25 °C. The resonant frequency is influenced by the viscosity and the density of the solution [29]. The frequencies of the quartz crystals (Stanford Research System) mounted in the holder made from Kynar and connected to an SRS QCM 100 unit were measured by a Philips PM 6685 frequency counter. The electrochemical measurements were performed with an Electroflex EF453 potentiostat.
Results and discussion
Effects of Bi(III) on the electrodeposition and reduction of PbO2 layers in nitrate media
Cyclic voltammetric study
EQCM experiments (Fig. 1 a and b) were carried out on gold-coated quartz crystal in 0.1 M Pb(NO3)2 dissolved in 1.0 M HNO3 solution containing different amounts of Bi(NO3)3 to study the behavior of the gold substrate and the electrodeposition process in these media.
The oxidation of lead(II) ion to lead dioxide started at high positive potentials (+ 1.5 V vs. SCE) during the anodic scan. The simultaneous frequency decrease indicated the deposition of the PbO2 layer. The effective molar mass for the oxidation process that involves the transfer of two electrons (n = 2) was found to be ca. 240 g mol−1. Then, this electrodeposited PbO2 layer was reduced during the cathodic scan (between + 1.3 and + 0.7 V vs. SCE) while the frequency almost returned to its initial value indicating the complete electrodissolution. The reduction of gold oxide was also experienced at + 0.9 V vs. SCE.
Increasing the amount of Bi(III) ion in the solution resulted in changes of the characteristics of cyclic voltammograms. A pair of peaks appeared at − 0.04 V vs. SCE which belongs to the reduction of Bi(III) to Bi(0) and the reverse reaction. The peak currents were proportional to the Bi(III) concentration. Based on the frequency changes, a reversible electrodissolution of the electrodeposited bismuth occurred, the frequency returned to its initial value. Regarding to these peaks, the effective molar masses (considering a 3e− transfer) were determined to be ca. 210 g mol−1 which is in a good agreement with the bismuth molar mass (209.0 g mol−1). The oxidation of lead(II) to lead dioxide started at higher positive potentials as well as the peak current decreased (smaller amount of PbO2 could be electrodeposited) as the amount of bismuth increased. It is also stated that smaller amount of gold oxide was reduced during the cathodic scan which is indicated by the decreasing peak currents at + 0.9 V vs. SCE. It means that the electrodeposition of lead dioxide from lead(II) nitrate acidic solution is less favorable if the electrolyte contains Bi(III). This phenomenon is explained by the adsorption of bismuth(III) ions on the oxidized gold surface [30] since those occupy the active spots on the surface of the substrate.
Chronoamperometric study
The electrodepositions of PbO2 layers were carried out by the previously developed method [18]. The chronoamperometric curves of the deposition from the solution containing 0.1 M Pb(NO3)2–1.0 M HNO3 and 0.05 M Bi(NO3)3–0.1 M Pb(NO3)2–1.0 M HNO3 are shown in Fig. 2 a and b, respectively. During the chronoamperometric deposition of lead dioxide, similar phenomenon has been experienced as described above; the deposition started at a higher positive potential (1.7 V instead of 1.6 V vs. SCE) (see Tables 1 and 2) because of bismuth adsorption on the oxidized gold surface. The effective molar masses calculated for a 2e− transfer process were found to be ca. 235 and 225 g mol−1 when the electrolyte did not contain Bi(III) and when it contained Bi(III), respectively. The lower molar mass value obtained is due to the lower utilization of current related to a partial delamination because of the worse adhesion between the gold oxide (hydroxide) substrate and the deposited PbO2 layer.
Chronoamperometric (solid lines) and simultaneous frequency (dashed lines) measurements during electrodeposition on gold-coated quartz crystal from the solution containing a 0.1 M Pb(NO3)2 and 1.0 M HNO3 and b 0.1 M Pb(NO3)2, 0.05 M Bi(NO3)3, and 1.0 M HNO3. The potential vs. time function is shown in Table 1 (a) and Table 2 (b), respectively
Electrochemical investigation of PbO2 layers modified by Bi(III) in sulfuric acid media
Characterization of PbO2 layers electrodeposited from Pb(II) and Bi(III) containing nitric acid solutions
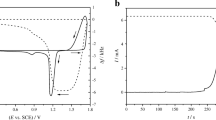
A lead dioxide layer was prepared by electrodeposition from the solution containing bismuth nitrate, lead nitrate, and nitric acid as shown in Fig. 2b. It was washed with double distilled water and then, it was put into 3.0 M sulfuric acid medium. The first three cyclic voltammograms were taken without using pre-oxidation step (Fig. 3a), but after that, the potential was held at + 1.8 V vs. SCE for 15 s (like a constant voltage charging of a battery) before starting each CV (Fig. 3b).
a The first three cyclic voltammograms (solid line) and simultaneous frequency measurements (dashed line) during the discharge of an electrodeposited PbO2 layer in 3.0 M H2SO4 solution. The scan rate was 50 mV s−1 in the potential range of + 1.8 to + 0.4 V vs. SCE. b Successive cyclic voltammograms obtained for the same layer. Before taking each CV, the potential was held at + 1.8 V vs. SCE for 15 s
The current and the frequency responses during the first three cycles were very similar to previously experienced ones [19]. During the successive cycles, the effective molar mass changes regarding to the transformation of PbO2 to PbSO4 were calculated to be ca. 63, 23, and 20 g mol−1, respectively (the difference between the molar mass of lead dioxide and lead sulfate is 64 g mol−1). It means that during the oxidation of lead(II) in nitrate media, almost totally crystalline β-PbO2 was electrodeposited; however, during the oxidation of lead sulfate (similarly to the charging of a lead-acid battery), a mixture of crystalline and amorphous (gelled, hydrated) material was formed. The hydrated lead dioxide can be described regarding to the second and third cycle as PbO2·xH2O or similarly PbO(OH)2·(x−1)H2O where x is ca. 2.3 and 2.4, respectively.
If pre-oxidation was applied before each cyclic voltammogram, two peaks appeared during the cathodic scan at ca. + 1.25 and + 1.35 V vs. SCE. The peak at lower potentials could be related to the presence of bismuth. This peak practically disappeared after ca. 7 cycles. Consequently, bismuth only affected the initial structure of PbO2 layer or Bi(III) adsorbed on the surface of lead dioxide influenced its electrochemical behavior.
Electrochemical study of PbO2 layers after adsorbing bismuth(III) ions in nitrate media
It has been described that bismuth(III) ions can adsorb on oxidized gold and platinum surfaces [30]; however, it is also possible that Bi3+ ions can adsorb on the electrodeposited PbO2, too. To investigate this process, lead dioxide was electrodeposited from the electrolyte containing 0.1 M Pb(NO3)2 and 1.0 M HNO3 solution by chronoamperometric method, then the layer was put into 1.0 M nitric acid. The open-circuit potential of the electrode and simultaneous frequency changes were measured while different amounts of 1.0 M HNO3–0.05 M Bi(NO3)3 solutions were added to the electrolyte (Fig. 4a).
a Measuring the potential of a lead dioxide electrode (solid line) and simultaneous frequency changes (dashed line) at open-circuit potential in 1.0 M HNO3 while adding different amounts of 1.0 M HNO3–0.05 M Bi(NO3)3 solutions to the electrolyte. b Estimated open-circuit potentials (filled squares) and frequency differences (empty squares) as a function of Bi(III) concentration
The open-circuit potential of the electrode exponentially decreased after adding Bi(III) into the solution until a mixture potential was reached. Simultaneously, the frequency curve showed a monotonous increase because of the chemical dissolution of PbO2 in acidic medium. A fast frequency decrease was observed just after the addition of each new portion of the Bi(III) ion containing solution. It was neglected in the further analysis since it is a usual artifact due to the functioning of the QCM technique. However, it can be seen that after each new addition, the frequency curve ran below the previous one although the frequency increase (mass decrease) was continuous. In order to analyze this effect, a straight line was fitted to each segment (e.g., between 12 and 14, 32 and 34, 46 and 48 min), then the frequency differences between the intercepts of the first and each parallel line were calculated. (See the supplementary material, Figs. S1 and S2.) These values and the estimated open-circuit potentials were plotted versus the concentration of bismuth(III) ion as shown in Fig. 4b. It can be seen that the OCP and frequency curves show a very good correlation. On the other hand, since the frequency curve resembles a Langmuir isotherm, it can be assumed that this effect is due to the adsorption of Bi(III).
After this treatment, the electrode was washed with double distilled water, then it was immersed in 3 M sulfuric acid solution. Except the first cycle, a pre-oxidation step was applied before starting the next cycle.
Similarly to the previously described case, two peaks appeared during the cathodic scan at ca. + 1.3 and + 1.4 V vs. SCE at the second cycle (Fig. 5a). The peak at lower potentials—which can be related to the presence of bismuth—disappeared after two cycles. Based on the cyclic voltammetric and simultaneous frequency measurements (Fig. 5b), it can be concluded that bismuth(III) in nitrate solution has almost negligible effect on the electrochemical behavior of lead dioxide while it is investigated later in sulfate solution. This phenomenon can be explained by that the bismuth—which is initially adsorbed on the surface of PbO2 layer—dissolves in the bulk solution during the reduction of lead dioxide in the first few cycles. However, the bulk concentration of Bi(III) is too low to influence the reduction/oxidation kinetics of lead dioxide/lead sulfate. When Bi(NO3)3 is introduced into the lead nitrate acidic solution during the electrodeposition, the influence of bismuth(III) on the PbO2 structure is significant as it has been previously experienced in methanesulfonate solutions [24].
Influence of Bi(III) on the charging-discharging and aging characteristics of lead dioxide
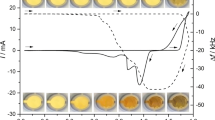
Two lead dioxide layers were electrodeposited from the solutions containing 0.1 M Pb(NO3)2 and 1.0 M HNO3, then both electrodes were washed with double distilled water before putting those into 3.7 M sulfuric acid solutions. The first three cyclic voltammograms for both electrodes were similar. After that, successive cyclic voltammograms were taken using a pre-oxidation step (holding the potential at + 1.8 V vs. SCE for 15 s before starting each CV) while different amounts of pure (Figs. 6a and 7a)/bismuth sulfate saturated (Figs. 6b and 7b) sulfuric acid solutions were added to the electrolyte.
Successive cyclic voltammograms obtained for an electrodeposited lead dioxide layer with the scan rate of 50 mV s−1. Before taking each CV, the potential was held at + 1.8 V vs. SCE for 15 s. The electrolyte was 3.7 M H2SO4 initially, then, different amounts of (a 3.7 M sulfuric acid and b 3.7 M sulfuric acid saturated with Bi2(SO4)3) solutions have been added to it
If the electrolyte did not contain Bi3+ ions, only two peaks appeared during the cathodic scan. The one at higher potentials is related to the transformation of lead dioxide to lead sulfate. The continuous increase of the cathodic peak current during cycling is due to the effect that more active material was involved in the redox transformations of PbO2/PbSO4. The other peak at lower positive potentials is related to the reduction of oxidized gold substrate where the cathodic peak current remained the same. With the increasing bismuth(III) concentration, a new peak appeared at ca. + 1.25 V vs. SCE, although the amount of charge which was transferred during the reduction of PbO2 (proportional to the sum of the areas of the two peaks at higher potentials) increased in the first seven cycles, then it decreased. In a real battery system with this charging method, it follows that the capacity of the positive electrode decreases after certain number of discharge-charge cycles while in the absence of bismuth, the capacity monotonously increases. This phenomenon can be assigned to the inhibiting effect of bismuth on the oxidation of lead sulfate (e.g., via reaction with OH radicals) or bismuth additive influences the reduction kinetics of PbO2 due to its adsorption.
When the electrolyte did not contain Bi(III), the effective molar mass change was ca. 25 g mol−1 at the first cycle and it decreased monotonously to ca. 10 g mol−1 until the last cycle. When the solution contained bismuth, the molar mass changes were ca. 15 and 30 g mol−1 at the first cycle and ca. − 10 and − 5 g mol−1 at the last cycle regarding to the peaks at + 1.4 and + 1.25 V vs. SCE, respectively. It is stated that the PbO2 reduction peak at lower potentials belongs to a more crystalline material and the peak at higher potentials belongs to a more hydrated one. It is also experienced that during the cyclization (aging of the layer), the effective molar mass changes decreased which means that the lead dioxide layer became more and more hydrated which is in good agreement with previous observations [19]. When the solution contained Bi(III), this process was observed much faster which indicates that bismuth accelerates the hydration of lead dioxide.
Understanding how bismuth influences the oxidation-reduction characteristics of PbO2
In order to better understand the influence of Bi3+ ions on the electrochemical behavior of lead dioxide, successive cyclic voltammograms (Fig. 8 a and b) were taken by varying the solution composition (pure sulfuric acid and sulfuric acid saturated with bismuth sulfate) during successive cycles as well as the peroxidation steps. The experimental conditions are summarized in Table 3.
a Successive cyclic voltammograms obtained for an electrodeposited lead dioxide layer in 3 M H2SO4 (the electrolyte also contained Bi(III) in some cases summarized in Table 5) with the scan rate of 50 mV s−1. Before taking each cyclic voltammogram, the potential was held at + 1.8 V vs. SCE for 15 s. b Simultaneous frequency measurements
When the electrolyte was pure sulfuric acid (1st cycle), a relatively narrow peak appeared with higher peak currents regarding to the reduction of lead dioxide. However, if the electrolyte used during cycling was changed to a Bi(III) containing one (2nd cycle), the peak potential was lower, ca. + 1.3 V instead of + 1.4 V vs. SCE. When the electrolyte was pure sulfuric acid during both the pre-oxidation and the polarization again (5th cycle), a small peak (shoulder) also appeared at ca. + 1.25 V vs. SCE which can be related to the residue of bismuth adsorbed on the surface of the lead dioxide layer. If the pre-oxidation was carried out in H2SO4 solution saturated with Bi2(SO4)3 (3rd and 4th cycles), cyclic voltammograms with significantly different shapes were obtained containing wide peaks (peak potentials were ca. + 1.3 V vs. SCE). It is also seen that the characteristics of the frequency curves changed significantly when sulfuric acid electrolyte saturated with bismuth sulfate was used during the pre-oxidation step for first time (3rd cycle). Based on these observations, it is evident that the bismuth content of the acid in which the pre-oxidation is carried out has a huge effect on the properties of the PbO2 layer. However, the presence of bismuth in the acid significantly influences the characteristics of the cyclic voltammetric curves only if the pre-oxidation was carried out in pure sulfuric acid. The most probable explanation for this phenomenon is that the bismuth adsorbs on the surface of the electrode and inhibits the oxidation of lead sulfate to lead dioxide. On the other hand, using bismuth containing sulfuric acid for the pre-oxidation step accelerates the hydration of the PbO2 layer during its aging.
Conclusions
Based on the elucidation of the results of the combined electrochemical and surface mass measurements as well as the effect of the solution composition, it is concluded that the presence of Bi3+ ions in the solution influences the structure of the PbO2 formed during the electrodeposition from nitric acid containing lead nitrate and bismuth nitrate. The bismuth also inhibits the oxidation of lead sulfate and affects the reduction kinetics of PbO2 in sulfuric acid media. In the course of successive cyclization (aging), the presence of bismuth accelerates the hydration of lead dioxide. These phenomena are related to the adsorption-desorption of bismuth on surfaces of PbO2 and the oxidized gold substrate, respectively.
References
Pavlov D (2011) Lead-acid batteries: science and technology. Elsevier, Oxford
Garche J, Karden E, Moseley PT, Rand DAJ (2017) Lead-acid batteries for future automobiles. Elsevier, Oxford
Karami H, Shamsipur M, Ghasemi S, Mousavi MF (2007) Lead–acid bipolar battery assembled with primary chemically formed positive pasted electrode. J Power Sources 164:896–904
Petersson I, Berghult B, Ahlberg E (1998) Thin lead dioxide electrodes for high current density applications in semi-bipolar batteries. J Power Sources 74:68–76
Johnson DC, Feng J, Houk LL (2000) Direct electrochemical degradation of organic wastes in aqueous media. Electrochim Acta 46:323–330
Chen S, Jiang F, Xie X, Zhou Y, Hu X (2016) Synthesis and application of lead dioxide nanowires for a PEM ozone generator. Electrochim Acta 192:357–362
Amadelli R, Armelao L, Velichenko AB, Nikolenko NV, Girenko DV, Kovalyov SV, Danilov FI (1999) Oxygen and ozone evolution at fluoride modified lead dioxide electrodes. Electrochim Acta 45:713–720
Pereira JF, Figueiredo RS, Ponce-de-León C, Bertazzoli R (2016) Platinum-free lead dioxide electrode for electrooxidation of organic compounds. J Solid State Electrochem 20:1167–1173
Hu X, Yu Y, Yang L (2015) Electrocatalytic activity of Ce-PbO2/C anode for acid red B reduction in aqueous solution. J Solid State Electrochem 19:1599–1609
Labiadh L, Barbucci A, Cerisola G, Gadri A, Ammar S, Panizza M (2015) Role of anode material on the electrochemical oxidation of methyl orange. J Solid State Electrochem 19:3177–3183
Zhou K, Tian Y, Ma H, Ma C, Fu Y, Dong X, Zhang X (2018) Photoelectrocatalytic performance of conductive carbon black-modified Ti/F-PbO2 anode for degradation of dye wastewater (reactive brilliant blue KN-R). J Solid State Electrochem 22:1131–1141
Zaidi SZJ, Harito C, Walsh FC, Ponce de León C (2018) Decolourisation of reactive black-5 at an RVC substrate decorated with PbO2/TiO2 nanosheets prepared by anodic electrodeposition. J Solid State Electrochem 22:2889–2900
Santos JEL, de Moura DC, da Silva DR, Panizza M, Martínez-Huitle CA (2019) Application of TiO2-nanotubes/PbO2 as an anode for the electrochemical elimination of Acid Red 1 dye. J Solid State Electrochem 23:351–360
Czerwiński A, Żelazowska M, Grdeń M, Kuc K, Milewski JD, Nowacki A, Wójcik G, Kopczyk M (2000) Electrochemical behavior of lead in sulfuric acid solutions. J Power Sources 85:49–55
Derafa I, Zerroual L, Matrakova M (2018) On the electrochemical activity of β-lead dioxide in sulfuric acid solution: a comparative study between the chemical and electrochemical routes. J Solid State Electrochem 22:1175–1183
Ruetschi P (2004) Aging mechanisms and service life of lead–acid batteries. J Power Sources 127:33–44
Oliveira CP, Lopes MC (2004) Early stages of the lead-acid battery discharge. J Power Sources 138:294–300
Broda B, Inzelt G (2018) Microgravimetric study of electrodeposition and dissolution of lead dioxide on gold and platinum substrates. J Solid State Electrochem 22:1–11
Broda B, Inzelt G (2020) Investigation of the electrochemical behaviour of lead dioxide in aqueous sulfuric acid solutions by using the in situ EQCM technique. J Solid State Electrochem 24:1–10
Chahmana N, Zerroual L, Matrakova M (2009) Influence of Mg2+, Al3+, Co2+, Sn2+ and Sb3+ on the electrical performance of doped β-lead dioxide. J Power Sources 191:144–148
Shmychkova O, Luk’yanenko T, Amadelli R, Velichenko A (2014) Physico-chemical properties of PbO2-anodes doped with Sn4+ and complex ions. J Electroanal Chem 717-718:196–201
Knysh V, Luk’yanenko T, Shmychkova O, Amadelli R, Velichenko A (2017) Electrodeposition of composite PbO2–TiO2 materials from colloidal methanesulfonate electrolytes. J Solid State Electrochem 21:537–544
Lam LT, Haigh NP, Rand DAJ (2000) Understanding the mechanism by which bismuth improves lead-acid battery capacity. J Power Sources 88:11–17
Shmychkova O, Luk’yanenko T, Velichenko A, Meda L, Amadelli R (2013) Bi-doped PbO2 anodes: electrodeposition and physico-chemical properties. Electrochim Acta 111:332–338
Koop MJ, Rand DAJ, Culpin B (1993) A guide to the influence of bismuth on lead/acid battery performance. J Power Sources 45:365–377
Sauerbrey G (1959) Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys 155:206–222
Taguchi M, Sugita H (2002) Analysis for electrolytic oxidation and reduction of PbSO4/Pb electrode by electrochemical QCM technique. J Power Sources 109:294–300
Pech D, Brousse T, Bélanger D, Guay D (2009) EQCM study of electrodeposited PbO2: investigation of the gel formation and discharge mechanisms. Electrochim Acta 54:7382–7388
Kanazawa KK, Gordon JG (1985) The oscillation frequency of a quartz resonator in contact with a liquid. Anal Chim Acta 175:99–105
Adžić RR, Marković NM (1985) Optical and electrochemical study of cation adsorption on oxide layers on gold and platinum electrodes. Electrochim Acta 30:1473–1479
Acknowledgements
Open access funding provided by Eötvös Loránd University (ELTE).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the eminent scientist and erudite scholar Professor Fritz Scholz on the occasion of his 65th birthday with the appreciation of his pioneering work in the field of solid state electrochemistry and numerous other contributions to contemporary electrochemistry
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Broda, B., Inzelt, G. Studying the effects of bismuth on the electrochemical properties of lead dioxide layers by using the in situ EQCM technique. J Solid State Electrochem 24, 2733–2739 (2020). https://doi.org/10.1007/s10008-020-04569-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04569-3