Abstract
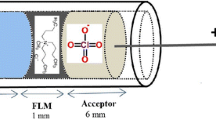
The determination of eugenol employing voltammetry of immobilized microdroplets (VIM) is reported in this work. The construction of the electrode was based on a glassy carbon substrate modified with carbon black nanoparticles and eugenol standard or clove oil sample within a dihexadecyl hydrogen phosphate (DHP) film. The different experimental parameters related to this proposed procedure, i.e., supporting electrolyte composition, pH, ionic strength, and square-wave voltammetry (SWV) parameters (amplitude, frequency, and step potential) were optimized. By doing that, the SWV measurements were performed on the following optimum experimental conditions: 0.2 mol L−1 phosphate buffer solution (pH 2.0), amplitude 90 mV, frequency 30 Hz, and step potential 5 mV. The analytical curve for eugenol was linear in the wide range from 0.03 to 26 μg (r = 0.999) with limits of detection (LOD) and quantification (LOQ) of 0.13 and 0.42 ng, respectively. Three commercial clove oil samples were successfully immobilized and analyzed using the proposed VIM procedure showing eugenol concentrations ranging from 14.0 to 68.3%. Comparing the results obtained by the proposed and comparative procedure (spectrophotometric), relative standard deviations (RSDs) lower than 5.0% were verified, demonstrating the concordance of the results obtained by both methods.









Similar content being viewed by others
References
Pubchem open chemistry database (2018) U.S. National Library of Medicine, Rockville. https://pubchem.ncbi.nlm.nih.gov/compound/eugenol. Accessed 1st Feb 2018
Schulz K, Schlenz K, Malt S, Metasch R, Römhild W, Dressler J, Lachenmeier DW (2008) Headspace solid-phase microextraction–gas chromatography–mass spectrometry for the quantitative determination of the characteristic flavouring agent eugenol in serum samples after enzymatic cleavage to validate post-offence alcohol drinking claims. J Chromatogr A 1211(1-2):113–119
Yildiz G, Aydogmus Z, Cinar ME, Senkal F, Ozturk T (2017) Electrochemical oxidation mechanism of eugenol on graphene modified carbon paste electrode and its analytical application to pharmaceutical analysis. Talanta 173:1–8
Kamatou GP, Vermaak I, Viljoen AM (2012) Eugenol—from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules 17(12):6953–6981
Jaganathan SK, Supriyanto E (2012) Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 17(12):6290–6304
Bolasina SN, Azevedo A, Petry AC (2017) Comparative efficacy of benzocaine, tricaine methanesulfonate and eugenol as anesthetic agents in the guppy Poecilia Vivipara. Aquacult Rep 6:56–60
Cowing D, Powell A, Johnson M (2015) Evaluation of different concentration doses of eugenol on the behaviour of Nephrops norvegicus. Aquaculture 442:78–85
Pisano M, Pagnan G, Loi M, Mura ME, Tilocca MG, Palmieri G, Fabbri D, Dettori MA, Delogu G, Ponzoni M, Rozzo C (2007) Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer 6(1):8–20
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2011) Pseudomonas resinovorans SPR1, a newly isolated strain with potential of transforming eugenol to vanillin and vanillic acid. New Biotechnol 28(6):656–664
Miyazawa M, Hisama M (2001) Suppression of chemical mutagen-induced SOS response by Alkylphenols from clove (Syzygium aromaticum) in the salmonella typhimurium TA1535/pSK1002 umu test. J Agric Food Chem 49(8):4019–4025
World Health Organization (1982) Geneva. http://apps.who.int/iris/bitstream/10665/41546/1/WHO_TRS_683.pdf. Accessed 1st Feb 2018
Ke C, Liu Q, Li L, Chen J, Wang X, Huang K (2016) Simultaneous determination of eugenol, isoeugenol and methyleugenol in fish fillet using gas chromatography coupled to tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 1031:189–194
Gopu CL, Aher S, Mehta H, Paradkar AR, Mahadik KR (2008) Simultaneous determination of cinnamaldehyde, eugenol and piperine by HPTLC densitometric method. Phytochem Anal 19(2):116–121
Dhoot G, Auras R, Rubino M, Dolan K, Soto-Valdez H (2009) Determination of eugenol diffusion through LLDPE using FTIR-ATR flow cell and HPLC techniques. Polymer 50(6):1470–1482
Saran S, Menon S, Shailajan S, Pokharna P (2013) Validated RP-HPLC method to estimate eugenol from commercial formulations like Caturjata Churna, Lavangadi Vati, Jatiphaladi Churna, Sitopaladi Churna and clove oil. J Pharm Res 6(1):53–60
Beaudry F, Guénette SA, Vachon P (2006) Determination of eugenol in rat plasma by liquid chromatography–quadrupole ion trap mass spectrometry using a simple off-line dansyl chloride derivatization reaction to enhance signal intensity. Biomed Chromatogr 20(11):1216–1222
Sağlam Ö, Dilgin DG, Ertek B, Dilgin Y (2016) Differential pulse voltammetric determination of eugenol at a pencil graphite electrode. Mater Sci Eng C 60:156–162
Yang L, Zhao F, Zeng B (2016) Electrochemical determination of eugenol using a three-dimensional molecularly imprinted poly (p-aminothiophenol-co-p-aminobenzoic acids) film modified electrode. Electrochim Acta 210:293–300
Feng Q, Duan K, Ye X, Lu D, Du Y, Wang C (2014) A novel way for detection of eugenol via poly (diallyldimethylammonium chloride) functionalized graphene-MoS2 nano-flower fabricated electrochemical sensor. Sensors Actuators B Chem 192:1–8
Wang LH, Chen JC (2011) Studies on the electrochemical behavior of eugenol and its application using a flow-through chronoamperometric sensor. Curr Pharm Anal 7(2):88–94
Afzali D, Zarei S, Fathirad F, Mostafavi A (2014) Gold nanoparticles modified carbon paste electrode for differential pulse voltammetric determination of eugenol. Mater Sci Eng C 43:97–101
Vicentini FC, Silva TA, Pellatieri A, Janegitz BC, Fatibello-Filho O, Faria RC (2014) Pb(II) determination in natural water using a carbon nanotubes paste electrode modified with crosslinked chitosan. Microchem J 116:191–196
Wang J (2005) Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17(1):7–14
Skrzypczyńska K, Kuśmierek K, Świątkowski A (2016) Carbon paste electrodes modified with various carbonaceous materials for the determination of 2,4-dichlorophenoxyacetic acid by differential pulse voltammetry. J Electroanal Chem 766:8–15
Arduini F, Majorani C, Amine A, Moscone D, Palleschi G (2011) Hg2+ detection by measuring thiol groups with a highly sensitive screen-printed electrode modified with a nanostructured carbon black film. Electrochim Acta 56(11):4209–4215
Arduini F, Di Nardo F, Amine A, Micheli L, Palleschi G, Moscone D (2012) Carbon black-modified screen-printed electrodes as electroanalytical tools. Electroanalysis 24(4):743–751
Silva TA, Fatibello-Filho O (2017) Square-wave adsorptive anodic stripping voltammetric determination of ramipril using an electrochemical sensor based on nanostructured carbon black. Anal Methods 9(32):4680–4687
Silva TA, Moraes FC, Janegitz BC, Fatibello-Filho O (2017) Electrochemical biosensors based on nanostructured carbon black: a review. J Nanomater 2017:1–14. https://doi.org/10.1155/2017/4571614
Janegitz BC, Baccarin M, Raymundo-Pereira PA, dos Santos FA, Oliveira GG, Machado SAS, Lanza MRV, Fatibello-Filho O, Zucolotto V (2015) The use of dihexadecylphosphate in sensing and biosensing. Sensors Actuators B Chem 220:805–813
Garcia LLC, Figueiredo-Filho LCS, Oliveira GG, Fatibello-Filho O, Banks CE (2013) Square-wave voltammetric determination of paraquat using a glassy carbon electrode modified with multiwalled carbon nanotubes within a dihexadecylhydrogenphosphate (DHP) film. Sensors Actuators B Chem 181:306–311
Ardila JA, Oliveira GG, Medeiros RA, Fatibello-Filho O (2013) Determination of gemfibrozil in pharmaceutical and urine samples by square-wave adsorptive stripping voltammetry using a glassy carbon electrode modified with multi-walled carbon nanotubes within a dihexadecyl hydrogen phosphate film. J Electroanal Chem 690:32–37
Vicentini FC, Janegitz BC, Brett CMA, Fatibello-Filho O (2013) Tyrosinase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and 1-butyl-3-methylimidazolium chloride within a dihexadecylphosphate film. Sensors Actuators B Chem 188:1101–1108
Maciel JV, Fava EL, Silva TA, Dias D, Fatibello-Filho O (2017) A combination of voltammetry of immobilized microparticles and carbon black-based crosslinked chitosan films deposited on glassy carbon electrode for the quantification of hydroquinone in dermatologic cream samples. J Solid State Electrochem 21(10):2859–2868
Scholz F, Nitschke L, Henrion G (1989) A new procedure for fast electrochemical analysis of solid materials. Naturwissenschaften 76(2):71–72
Scholz F, Nitschke L, Henrion G, Damaschun F (1989) A technique to study the electrochemistry of minerals. Naturwissenschaften 76(4):167–168
Doménech-Carbó A, Labuda J, Scholz F (2012) Electroanalytical chemistry for the analysis of solids: characterization and classification (IUPAC technical report). Pure Appl Chem 85:609–631
Doménech A, Doménech-Carbó MT, Martínez-Lázaro I (2010) Layer-by-layer identification of copper alteration products in metallic works of art using the voltammetry of microparticles. Anal Chim Acta 680(1-2):1–9
Di Turo F, Montoya N, Piquero-Cilla J, De Vito C, Coletti F, Favero G, Doménech-Carbó A (2017) Archaeometric analysis of roman bronze coins from the magna mater temple using solid-state voltammetry and electrochemical impedance spectroscopy. Anal Chim Acta 955:36–47
Doménech-Carbó A, Doménech-Carbó MT, Valle-Algarra FM, Gimeno-Adelanrado JV, Bosch-Reig F (2016) On-line database of voltammetric data of immobilized particles for identifying pigments and minerals in archaeometry, conservation and restoration (ELCHER database). Anal Chim Acta 927:1–12
Scholz F, Schröder U, Gulaboski R (2005) Electrochemistry of immobilized particles and droplet. Springer, Berlin
Scholz F, Schröder U, Gulaboski R, Doménech-Carbó A (2014) Electrochemistry of immobilized particles and droplets: experiments with three-phase electrodes. 2014, Springer, Berlin
Wadhawan JD, Evans RG, Compton RG (2002) Voltammetric characteristics of graphite electrodes modified with microdroplets of n-butylferrocene. J Electroanal Chem 533(1-2):71–84
Munoz RA, Banks CE, Davies TJ, Angnes L, Compton RG (2006) The electrochemistry of tetraphenyl porphyrin iron (III) within immobilized droplets supported on platinum electrodes. Electroanalysis 18(7):649–654
Liu Z, Wang Z, Cao Y, Jing Y, Liu Y (2011) High sensitive simultaneous determination of hydroquinone and catechol based on graphene/BMIMPF6 nanocomposite modified electrode. Sensors Actuators B Chem 157(2):540–546
Nematollahi D, Shayani-Jam H, Alimoradi M, Niroomand S (2009) Electrochemical oxidation of acetaminophen in aqueous solutions: kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochim Acta 54(28):7407–7415
Brett AMO, Brett CMA (1996) Electroquímica: Princípios, Métodos e Aplicações. Livraria Almedina, Coimbra
Resolução- RCD N° 3 (2012) Agência Nacional de Vigilância Sanitária (ANVISA), BRASIL. http://www.vigilanciasanitaria.sc.gov.br/index.php/download/category/126-cosmeticos-produtos-de-higiene-pessoal-e-perfumes?download=1676:rdc-03-2012-lista-de-substancia-que-os-produtos-nao-podem-apresentar-exceto-mercosul. Accessed 1st Feb 2018
Acknowledgments
We would like to thank CEME-SUL FURG and CAB English Lessons for the English correction.
Funding
We gratefully acknowledge the financial support of the following Brazilian Funding Agencies CNPq (444150/2014-5) and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maciel, J.V., Silva, T.A., Dias, D. et al. Electroanalytical determination of eugenol in clove oil by voltammetry of immobilized microdroplets. J Solid State Electrochem 22, 2277–2285 (2018). https://doi.org/10.1007/s10008-018-3933-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3933-z