Abstract
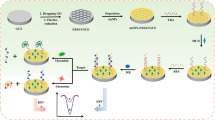
In this study, a functionalized nanocomposite-based electrochemiluminescence (ECL) sensor for detecting thrombin was developed. First, Ru(bpy)32+/β-cyclodextrin-Au nanoparticles (β-CD-AuNPs)/nanographene (NGP) composites were used to modify the glassy carbon electrode (GCE) surface, and then aptamers (TBA1 and TBA2 with a 1:1 M ratio) were labeled with ferrocene (Fc) acting as the probes and were attached to the composite via the host–guest recognition between β-CD and Fc. In the absence of thrombin, the quenching of Fc to [Ru(bpy)3]2+ was maintained, and “signal-off” ECL was observed. However, because of the specific combination of the aptamer probes and thrombin, the configuration of aptamer probes changed and escaped from the electrode surface once thrombin appears, which results in the quenching disappearance, and the ECL signal was changed from “off” to “on.” Meanwhile, the application of nanocomposites amplified the effect of “signal-on.” By this strategy, thrombin was detected with high sensitivity and with a detection limit down to 0.23 pM. Moreover, the relatively simple ECL sensor exhibited excellent reproducibility with at least 6 cycles of recovering the original signal.








Similar content being viewed by others
References
Archiniegas E, Neves CY, Candelle D, Cardier JE (2004) Thrombin and its protease-activated receptor-1 (PAR1) participate in the endothelial-mesenchymal transdifferentiation process. DNA Cell Biol 23(12):815–825
Popovic M, Smiljanic K, Dobutovic B, Syrovets T, Simmet T, Isenovic ER (2012) Thrombin and vascular inflammation. Mol Cell Biochem 359(1-2):301–313
Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S (2004) Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood 104(9):2746–2751
Nierodzik ML, Karpatkin S (2006) Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 10(5):355–362
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Kandimalla VB, Ju HX (2004) New horizons with a multi dimensional tool for applications in analytical chemistry—aptamer. Anal Lett 37(11):2215–2233
Patel DJ, Suri AK (2000) Structure, recognition and discrimination in RNA aptamer complexes with cofactors, amino acids, drugs and aminoglycoside antibiotics. J Biotechnol 74:39–60
Wu ZS, Zheng F, Shen GL, Yu RQ (2009) A hairpin aptamer-based electrochemical biosensing platform for the sensitive detection of proteins. Biomaterials 30(15):2950–2955
Cui HF, Cheng L, Zhang J, Liu RH, Zhang C, Fan H (2014) An electrochemical DNA sensor for sequence-specific DNA recognization in a homogeneous solution. Biosens Bioelectron 56:124–128
Hong N, Cheng L, Wei GB, Chen CD, He LL, Kong DL, Ceng JX, Cui HF, Fan H (2017) An electrochemical DNA. Sensor without electrode pre-modification. Biosens Bioelectron 91:110–114
Zhu X, Zhang YS, Yang WQ, Liu QD, Lin ZY, Qiu B, Chen GN (2011) Highly sensitive electrochemiluminescent biosensor for adenosine based on structure-switching of aptamer. Anal Chim Acta 684(1-2):121–125
Alpuche-Aviles MA, Wipf DO (2001) Impedance feedback control for scanning electrochemical microscopy. Anal Chem 73(20):4873–4881
Zhang MH, Yuan R, Chai YQ, Chen SH, Zhong HA, Wang C, Cheng YF (2012) A biosensor for cholesterol based on gold nanoparticles-catalyzed luminol electrogenerated chemiluminescence. Biosens Bioelectron 32(1):288–292
Wang HJ, Bai LJ, Chai YQ, Yuan R (2014) Synthesis of multi-fullerenes encapsulated palladium nanocage, and its application in electrochemiluminescence immunosensors for the detection of streptococcus suis serotype 2. Small 10:1857–1865
Wang XY, Gao A, Lu CC, He XW, Yin XB (2013) An electrochemiluminescence aptasensor for thrombin using graphene oxide to immobilize the aptamer and the intercalated Ru(phen)(3)(2+) probe. Biosens Bioelectron 48:120–125
Fang LY, Lv ZZ, Wei H, Wang EK (2008) A electrochemiluminescence aptasensor for detection of thrombin incorporating the capture aptamer labeled with gold nanoparticles immobilized onto the thio-silanized ITO electrode. Anal Chim Acta 628(1):80–86
Chen Q, Chen H, Zhao YY, Zhang F, Yang F, Tang J, He PG (2014) A label-free electrochemiluminescence aptasensor for thrombin detection based on host-guest recognition between tris(bipyridine) ruthenium(II)-beta-cyclodextrin and aptamer. Biosens Bioelectron 54:547–552
Zhang J, Chen PP, Wu XY, Chen JH, Xu LJ, Chen GN, Fu FF (2011) A signal-on electrochemiluminescence aptamer biosensor for the detection of ultratrace thrombin based on junction-probe. Biosens Bioelectron 26(5):2645–2650
Zhuo BR, Li YQ, Huang X, Lin YJ, Chen YW, Gao WH (2015) An electrochemiluminescence aptasensing platform based on ferrocene-graphene nanosheets for simple and rapid detection of thrombin. Sensors Actuators B Chem 208:518–524
Numnuam A, Chumbimuni-Torres KY, Xiang Y, Bash R, Thavarungkul P, Kanatharana P, Pretsch E, Wang J, Bakker E (2008) Aptamer-based potentiometric measurements of proteins using ion-selective microelectrodes. Anal Chem 80(3):707–712
Li YJ, Li YQ, Xu N, Pan JH, Chen TF, Chen YW, Gao WH (2017) Dual-signal amplification strategy for electrochemiluminescence sandwich biosensor for detection of thrombin. Sensors Actuators B Chem 240:742–748
Jie GF, Lu ZK, Zhao Y, Wang XC (2017) Quantum dots bilayers/Au@Ag-based electrochemiluminescence resonance energy transfer for detection of thrombin by autocatalytic multiple amplification strategy. Sensors Actuators B Chem 240:857–862
Liu YM, Shi GF, Zhang JJ, Zhou M, Cao JT, Huang KJ, Ren SW (2014) A novel label-free electrochemiluminescence aptasensor based on layered flowerlike molybdenum sulfide-graphene nanocomposites as matrix. Colloid Surface B 122:287–293
Wang J, Jiang XC, Han HY (2016) Turn-on near-infrared electrochemiluminescence sensing of thrombin based on resonance energy transfer between CdTe/CdS core(small)/shell(thick) quantum dots and gold nanorods. Biosens Bioelectron 82:26–31
Yu XX, Cui H (2014) Electrochemiluminescence bioassay for thrombin based on dynamic assembly of aptamer, thrombin and N-(aminobutyl)-N-(ethylisoluminol) functionalized gold nanoparticles. Electrochim Acta 125:156–162
Liu YM, Zhang JJ, Shi GF, Zhou M, Liu YY, Huang KJ, Chen YH (2014) Label-free electrochemiluminescence aptasensor using Ru(bpy)(3)(2+) functionalized dopamine-melanin colloidal nanospheres and gold nanoparticles as signal-amplifying tags. Electrochim Acta 129:222–228
Bouhadiba A, Belhocine Y, Rahim M, Djilani I, Nouar L, Khatmi DE (2017) Host-guest interaction between tyrosine and (beta-cyclodextrin): molecular modeling and nuclear studies. J Mol Liq 233:358–363
Wu LD, Deng DH, Jin J, Lu XB, Chen JP (2012) Nano-graphene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens Bioelectron 35(1):193–199
Ha W, Kang Y, Peng SL, Ding LS, Zhang S, Li BJ (2013) Vesicular gold assemblies based on host-guest inclusion and its controllable release of doxorubicin. Nanotechnology 24(49):495103
Patolsky F, Lichtenstein A, Willner I (2000) Amplified microgravimetric quartz-crystal-microbalance assay of DNA using oligonucleotide-functionalized liposomes or biotinylated liposomes. J Am Chem Soc 122(2):418–419
Chen D, Tang LH, Li JH (2010) Graphene-based materials in electrochemistry. Chem Soc Rev 39(8):3157–3180
Wang XY, Dong P, Yun W, Xu Y, He PG, Fang YZ (2010) Detection of T4 DNA ligase using a solid-state electrochemiluminescence biosensing switch based on ferrocene-labeled molecular beacon. Talanta 80(5):1643–1647
Shangguan DH, Meng L, Cao ZC, Xiao ZY, Fang XH, Li Y, Cardona D, Witek RP, Liu C, Tan WH (2008) Identification of liver cancer-specific aptamers using whole live cells. Anal Chem 80(3):721–728
Pavlov V, Xiao Y, Shlyahovsky B, Willner I (2004) Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J Am Chem Soc 38:11768–11769
Fan H, Chang Z, Xing R, Chen M, Wang QJ, He PG, Fang YZ (2008) An electrochemical aptasensor for detection of thrombin based on target protein-induced strand displacement. Electroanalysis 20(19):2113–2117
Siegel B, Breslow R (1975) Lyophobic binding of substrates by cyclodextrins in nonaqueous solvents. J Am Chem Soc 97(23):6869–6870
Centi S, Messina G, Tombelli S, Palchetti I, Mascini M (2008) Different approaches for the detection of thrombin by an electrochemical aptamer-based assay coupled to magnetic beads. Biosens Bioelectron 23(11):1602–1609
Acknowledgements
This work was kindly supported by the National Natural Science Foundation of China (81660658) and (81560625), JiangXi Science and Education Committee (GJJ160816) and (GJJ160853). Jiangxi University of Traditional Chinese Medicine Innovation Foundation (JZYC18S12).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, C., Wei, G., Yao, X. et al. Ru(bpy)32+/β-cyclodextrin-Au nanoparticles/nanographene functionalized nanocomposites-based thrombin electrochemiluminescence aptasensor. J Solid State Electrochem 22, 2059–2066 (2018). https://doi.org/10.1007/s10008-018-3910-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3910-6