Abstract
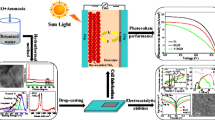
Fe3O4-reduced graphene oxide (Fe3O4-RGO) binder-free counter electrode (CE) is prepared by using an easy and low-cost electrophoretic deposition method and controlling the hydrogen evolution process followed by an electrochemical reduction process for dye-sensitized solar cell (DSSC). X-ray diffraction, X-ray photoelectron spectroscopy, energy dispersive spectrometer, Raman spectroscopy, field emission scanning electron microscopy, and transmission electron microscopy (TEM) indicate clearly the formation of Fe3O4-RGO nanocomposite. TEM images show that the Fe3O4 nanoparticles with diameters in the range of 10–30 nm are uniformly deposited on RGO. The layer-by-layer deposition of iron oxide species anchored on graphene nanosheets during the EPD on FTO provides a unique film for DSSC. To evaluate the chemical catalysis and stability of prepared CEs toward I3 − reduction and the interfacial charge transfer properties, Fe3O4-RGO nanocomposite and RGO are characterized by cyclic voltammetry, Tafel polarization, and electrochemical impedance spectroscopy. Under AM 1.5 irradiation (100 mW cm−2), the DSSC based on the Fe3O4-RGO shows a power conversion efficiency of 5.91%, which is comparable with the Pt CE, suggesting that the Fe3O4-RGO nanocomposite is an effective CE material for low-cost DSSC. The proposed approach can prepare a thin film of Fe3O4-RGO at short time with suitable performance in DSSC.

Graphical abstract








Similar content being viewed by others
References
Hagfeldt A, Boschloo G, Sun L, Kloo L, Petterssn H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Yang W, Ma X, Xu X, Li Y, Raj S, Ning G, Wang A, Chen SH (2015) Sulfur-doped porous carbon as metal-free counter electrode for high-efficiency dye-sensitized solar cells. J Power Sources 282:228–234
Wu M, Ma T (2014) Recent progress of counter electrode catalysts in dye-sensitized solar cells. J Phys Chem C 118:16727–16742
Jinbiao J, Jihuai W, Yongguang T, Jinghao H, Min Z, Jianming L (2015) Transparent nickel selenide used as counter electrode in high efficient dye-sensitized solar cells. J Alloys Compd 640:29–33
O’Reagen B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–74+0
Li P, Wu J, Lin J, Huang M, Huang Y, Li Q (2009) High-performance and low platinum loading Pt/carbon black counter electrode for dye-sensitized solar cells. Sol Energy 83:845–849
Velten J, Mozer AJ, Li D, Officer D, Wallace G, Baughman R, Zakhidov A (2012) Carbon nanotube/graphene nanocomposite as efficient counter electrodes in dye-sensitized solar cells. Nanotechnology 23:085201
Dao VD, Larina LL, Lee JK, Jung KD, Huy BT, Choi HS (2015) Graphene-based RuO2 nanohybrid as a highly efficient catalyst for triiodide reduction in dye-sensitized solar cells. Carbon 81:710–719
Yao Y, Miao S, Liu S, Ma LP, Sun H, Wang S (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332
Kyotani T, Tsai L, Tomita A (1997) Formation of platinum nanorods and nanoparticles in uniform carbon nanotubes prepared by a template carbonization method. Chem Commun 4756:701–702
Flahaut E, Peigney A, Laurent C, Marlière C, Chastel F, Rousset A (2000) Carbon nanotube–metal–oxide nanocomposites: microstructure, electrical conductivity and mechanical properties. Acta Mater 48:3803–3812
Van der Zaag P, Bloemen P (2000) On the construction of an Fe3O4-based all-oxide spin valve. J Magn Magn Mater 211:301–308
Raj K, Moskowitz B, Casciari R (1995) Advances in ferrofluid technology. J Magn Magn Mater 149:174–180
Tahir AA, Upul Wijayantha KG, Saremi-Yarahmadi S, Maznar M, Mckee V (2009) Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation. Chem Mater 21:3763–3772
Deosarkar MP, Pawar SM, Bhanvase BA (2014) In situ sonochemical synthesis of Fe3O4-graphene nanocomposite for lithium rechargeable batteries. Chem Eng Process Process Intensif 83:49–55
Li L, Gao P, Gai S, He F, Chen Y, Zhang M, Yang P (2016) Ultra small and highly dispersed Fe3O4 nanoparticles anchored on reduced graphene for supercapacitor application. Electrochim Acta 190:566–573
Ding Y, Shen SZ, Sun H, Sun K, Liu F, Qi Y, Yan J (2015) Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater Sci Eng C 48:487–498
Houmad M, Zaari H, Benyoussef A, El Kenz A, Ez-Zahraouy H (2015) Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 94:1021–1027
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
An SJ, Zhu Y, Lee SH, Stoller MD, Emilsson T, Park S, Velamakanni A, An J, Ruoff R (2010) Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition. J Phys Chem Lett 1:1259–1263
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Huo J, Zheng M, Tu Y, Wu J, Hu L, Dai S (2015) A high performance cobalt sulfide counter electrode for dye-sensitized solar cells. Electrochim Acta 159:166–173
Sarkar P, De D, Uchikochi T, Besra L (2012) Electrophoretic deposition (EPD): fundamentals and novel applications in fabrication of advanced ceramic microstructures. Electrophor Depos Nanomater 52:190
Battumur T, Mujawar SH, Truong QT, Ambade SB, Lee DS, Lee W, Han SH (2012) Graphene/carbon nanotubes composites as a counter electrode for dye-sensitized solar cells. Curr Appl Phys 12:49–53
Dou YY, Li GR, Song J, Gao XP (2012) Nickel phosphide-embedded graphene as counter electrode for dye-sensitized solar cells. Phys Chem Chem Phys 14:1339–1342
Yue G, Lin JY, Tai SY, Xiao Y, Wu J (2012) A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim Acta 85:162–168
William J, Hummers S, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Wu Z, Pei S, Ren W, Tang D, Gao L, Liu B, Cheng H (2009) Field emission of single-layer graphene films prepared by electrophoretic deposition. Adv Mater 21:1756–1760
Lian P, Zhu X, Xiang H, Li Z, Yang W, Wang H (2010) Enhanced cycling performance of Fe3O4-graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim Acta 56:834–840
Salamon J, Sathishkumar Y, Ramachandran K, Soo Y, Jin D, Rhan A, Gnana G (2015) One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens Bioelectron 64:269–276
Cao K, Jiao L, Liu H, Liu Y, Wang Y, Guo Z, Yuan H (2015) 3D hierarchical porous α-Fe2O3 nanosheets for high-performance lithium-ion batteries. Adv Energy Mater 5:1–9
Izaki M, Shinoura O (2001) Room-temperature deposition of defect-free magnetite film by chemical reaction from an aqueous solution. Adv Mater 13:142–145
Peng S, Zhu P, Thavasi V, Mhaisalkar SG, Ramakrishna S (2011) Facile solution deposition of ZnIn2S4 nanosheet films on FTO substrates for photoelectric application †. Nano 3:2602–2608
Nethravathi C, Rajamathi M (2008) Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 46:1994–1998
Ghasemi S, Ahmadi F (2015) Effect of surfactant on the electrochemical performance of graphene/iron oxide electrode for supercapacitor. J Power Sources 289:129–137
Zhang M, Jia MQ, Jin YH (2012) Fe3O4/reduced graphene oxide nanocomposite as high performance anode for lithium ion batteries. Appl Surf Sci 261:298–305
Li X, Huang X, Liu D, Wang X, Song S, Zhou L, Zhang H (2011) Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J Phys Chem C 115:21567–21573
Sun G, Dong B, Cao M, Wei B, Hu C (2011) Hierarchical dendrite-like magnetic materials of Fe3O4, γ-Fe2O3, and Fe with high performance of microwave absorption. Chem Mater 23:1587–1593
Li X, Si Z, Lei Y, Tang J, Wang S, Su S, Song S, Zhao L, Zhang H (2010) Direct hydrothermal synthesis of single-crystalline triangular Fe3O4 nanoprisms. Cryst Eng Comm 12:2060
Bhuvaneswari S, Pratheeksha PM, Anandan S, Rangappa D, Gopalan R, Rao TN (2014) Efficient reduced graphene oxide grafted porous Fe3O4 composite as a high performance anode material for Li-ion batteries. Phys Chem Chem Phys 16:5284
Liu L, Zhao F, Liu J, Yang F (2013) Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR. J Memb Sci 437:99–107
Mane RS, Chang J, Ham D, Pawar BN, Ganesh T, Cho BW, Lee JK, Han SH (2009) Dye-sensitized solar cell and electrochemical supercapacitor applications of electrochemically deposited hydrophilic and nanocrystalline tin oxide film electrodes. Curr Appl Phys 9:87–91
Zhu Y, Xu X, Zhang L, Chen J, Cao Y (2012) High efficiency inverted polymeric bulk-heterojunction solar cells with hydrophilic conjugated polymers as cathode interlayer on ITO. Sol Energy Mater Sol Cells 97:83–88
Lee TH, Do K, Lee YW, Jeon SS, Kim C, Ko J, Im S (2012) High-performance dye-sensitized solar cells based on PEDOT nanofibers as an efficient catalytic counter electrode. J Mater Chem 22:21624
Bi H, Cui H, Lin T, Huang F (2015) Graphene wrapped copper–nickel nanospheres on highly conductive graphene film for use as counter electrodes of dye-sensitized solar cells. Carbon 91:153–160
Hauch A, Georg A (2001) Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim Acta 46:3457–3466
Zhang J, Ma M, Tang Q, Yu L (2016) Multistep electrochemical deposition of hierarchical platinum alloy counter electrodes for dye-sensitized solar cells. J Power Sources 303:243–249
Liao Y, Pan K, Wang L, Pan Q, Zhou W, Miao X, Fu H (2013) Facile synthesis of high-crystallinity graphitic carbon/Fe3C nanocomposites as counter electrodes for high-efficiency dye-sensitized solar cells. ACS Appl Mater 5:3663–3670
Xiao Y, Wang C, Han G (2015) Effects of thiourea concentration on electrocatalytic performances of nickel sulfide counter electrodes for use in dye-sensitized solar cells. Mater Res Bull 61:326–332
Chang Q, Ma Z, Wang J, Yan Y, Shi W, Chen Q (2015) Graphene nanosheets@ZnO nanorods as three-dimensional high efficient counter electrodes for dye sensitized solar cells. Electrochim Acta 151:459–466
Wang H, Sun K, Tao F, Stacchiola DJ, Hu YH (2013) 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells. Angew Chemie - Int Ed 52:9210–9214
Wang H, Wei W, Hu YH (2014) NiO as an efficient counter electrode catalyst for dye-sensitized solar cells. Top Catal 57:607–611
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, S., Hosseini, S.R. & Kazemi, Z. Electrophoretic preparation of graphene-iron oxide nanocomposite as an efficient Pt-free counter electrode for dye-sensitized solar cell. J Solid State Electrochem 22, 245–253 (2018). https://doi.org/10.1007/s10008-017-3741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3741-x