Abstract
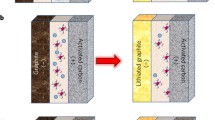
The integration of a battery-type electrode and of a capacitor-type electrode in a single device by proper design is an effective strategy in developing energy storage devices with high energy and power densities. Herein, we present a battery-supercapacitor hybrid device using metallic zinc as anode, a biodegradable ionic liquid (IL) as electrolyte, and graphite as cathode. The recently developed choline acetate ([Ch]OAc) biodegradable IL-based electrolyte enables reversible deposition/stripping of Zn(II). Spongy-like Zn with a high surface area is obtained, which allows fast charge/discharge at high rates. The adsorption/desorption of ions on the surface of the graphite cathode and intercalation/deintercalation of anions into/from the graphite layers occur at the graphite cathode. Raman spectra and X-ray photoelectron reveal the intercalation of IL into and the adsorption of IL on the graphite. Highly reversible adsorption/desorption of ions on the surface of the graphite electrodes in the [Ch]OAc-based electrolyte was demonstrated by a symmetric cell. The Zn/graphite hybrid device delivers an energy density of 53 Wh kg−1 at a power density of ~ 145 W kg−1 and 42 Wh kg−1 at ~ 400 W kg−1. The hybrid device also exhibits a long cycle life with ∼ 86% specific capacitance retained after 1000 cycles at a current density of 0.5 A g−1. The combination of well-available zinc, inexpensive graphite, and a biodegradable IL electrolyte in a cell could open new avenues for sustainable energy applications.

ᅟ







Similar content being viewed by others
References
Luo X, Wang J, Dooner M, Clarke J (2015) Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl Energy 137:511–536
Chen H, Cong TN, Yang W, Tan C, Li Y, Ding Y (2009) Progress in electrical energy storage system: a critical review. Prog Nat Sci 19:291–312
Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Cabrera-Castillo E, Niedermeier F, Jossen A (2016) Calculation of the state of safety (SOS) for lithium ion batteries. J Power Sources 324:509–520
Zuo W, Li R, Zhou C, Li Y, Xia J, Liu J (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 1600539
Dubal DP, Ayyad O, Ruiz V, Gomez-Romero P (2015) Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem Soc Rev 44:1777–1790
Aravindan V, Reddy MV, Madhavi S, Mhaisalkar SG, Subba Rao GV, Chowdari BVR (2011) Hybrid supercapacitor with nano-TiP2O7 as intercalation electrode. J Power Sources 196:8850–8854
Vijayan S, Kirubasankar B, Pazhamalai P, Solarajan AK, Angaiah S (2017) Electrospun Nd3+-doped LiMn2O4 nanofibers as high-performance cathode material for Li-ion capacitors. ChemElectroChem DOI. doi:10.1002/celc.201700161
Aravindan V, Chuiling W, Reddy MV, Rao GVS, Chowdari BVR, Madhavi S (2012) Carbon coated nano-LiTi2(PO4)3 electrodes for non-aqueous hybrid supercapacitors. Phys Chem Chem Phys 14:5808–5814
Flora XH, Ulaganathan M, Babu RS, Rajendran S (2012) Evaluation of lithium ion conduction in PAN/PMMA-based polymer blend electrolytes for Li-ion battery applications. Ionics 18:731–736
Aswathy R, Kesavan T, Kumaran KT, Ragupathy P (2015) Octahedral high voltage LiNi0.5Mn1.5O4 spinel cathode: enhanced capacity retention of hybrid aqueous capacitors with nitrogen doped graphene. J Mater Chem A 3:12386–12395
Kirubasankar B, Murugadoss V, Angaiah S (2017) Hydrothermal assisted in situ growth of CoSe onto graphene nanosheets as a nanohybrid positive electrode for asymmetric supercapacitors. RSC Adv 7:5853–5862
Arun N, Jain A, Aravindan V, Jayaraman S, Chui Ling W, Srinivasan MP, Madhavi S (2015) Nanostructured spinel LiNi0.5Mn1.5O4 as new insertion anode for advanced Li-ion capacitors with high power capability. Nano Energy 12:69–75
Kumar M, Subramania A, Balakrishnan K (2014) Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim Acta 149:152–158
Zuo W, Wang C, Li Y, Liu J (2015) Directly grown nanostructured electrodes for high volumetric energy density binder-free hybrid supercapacitors: a case study of CNTs//Li4Ti5O12. Sci Rep 5:7780
Hu X, Deng Z, Suo J, Pan Z (2009) A high rate, high capacity and long life (LiMn2O4 + AC)/Li4Ti5O12 hybrid battery–supercapacitor. J Power Sources 187:635–639
Liu X, Jung H-G, Kim S-O, Choi H-S, Lee S, Moon JH, Lee JK (2013) Silicon/copper dome-patterned electrodes for high-performance hybrid supercapacitors. Sci Rep 3:3183
Kolathodi MS, Palei M, Natarajan TS (2015) Electrospun NiO nanofibers as cathode materials for high performance asymmetric supercapacitors. J Mater Chem A 3:7513–7522
Yan J, Fan Z, Sun W, Ning G, Wei T, Zhang Q, Zhang R, Zhi L, Wei F (2012) Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv Funct Mater 22:2632–2641
Perret P, Khani Z, Brousse T, Bélanger D, Guay D (2011) Carbon/PbO2 asymmetric electrochemical capacitor based on methanesulfonic acid electrolyte. Electrochim Acta 56:8122–8128
Kim D, Kang S-H, Slater M, Rood S, Vaughey JT, Karan N, Balasubramanian M, Johnson CS (2011) Enabling sodium batteries using lithium-substituted sodium layered transition metal oxide cathodes. Adv Energy Mater 1:333–336
Yabuuchi N, Kajiyama M, Iwatate J, Nishikawa H, Hitomi S, Okuyama R, Usui R, Yamada Y, Komaba S (2012) P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat Mater 11:512–517
Ramya R, Sivasubramanian R, Sangaranarayanan MV (2013) Conducting polymers-based electrochemical supercapacitors—progress and prospects. Electrochim Acta 101:109–129
Frackowiak E, Béguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39:937–950
Zhang Y, Feng H, Wu X, Wang L, Zhang A, Xia T, Dong H, Li X, Zhang L (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrog Energy 34:4889–4899
Galiński M, Lewandowski A, Stępniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51:5567–5580
Mousavi MPS, Wilson BE, Kashefolgheta S, Anderson EL, He S, Bühlmann P, Stein A (2016) Ionic liquids as electrolytes for electrochemical double-layer capacitors: structures that optimize specific energy. ACS Appl Mater Interfaces 8:3396–3406
MacFarlane DR, Forsyth M, Howlett PC, Kar M, Passerini S, Pringle JM, Ohno H, Watanabe M, Yan F, Zheng W, Zhang S, Zhang J (2016) Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat Rev Mater 1:15005
Armand M, Endres F, MacFarlane DR, Ohno H, Scrosati B (2009) Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater 8:621–629
Kundu D, Adams BD, Duffort V, Vajargah SH, Nazar LF (2016) A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat Energy 1:16119
Liu Z, Pulletikurthi G, Endres F (2016) A Prussian blue/zinc secondary battery with a bio-ionic liquid–water mixture as electrolyte. ACS Appl Mater Interfaces 8:12158–12164
Gu P, Zheng M, Zhao Q, Xiao X, Xue H, Pang H (2017) Rechargeable zinc-air batteries: a promising way to green energy. J Mater Chem A 5:7651–7666
Fu J, Cano ZP, Park MG, Yu A, Fowler M, Chen Z (2017) Electrically rechargeable zinc–air batteries: progress, challenges, and perspectives. Adv Mater 29:1604685
Li Y, Gong M, Liang Y, Feng J, Kim J-E, Wang H, Hong G, Zhang B, Dai H (2013) Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat Commun 4:1805
Parker JF, Chervin CN, Pala IR, Machler M, Burz MF, Long JW, Rolison DR (2017) Rechargeable nickel–3D zinc batteries: an energy-dense, safer alternative to lithium-ion. Science 356:415–418
Yoo HD, Han S-D, Bayliss RD, Gewirth AA, Genorio B, Rajput NN, Persson KA, Burrell AK, Cabana J (2016) “Rocking-chair”-type metal hybrid supercapacitors. ACS Appl Mater Interfaces 8:30853–30862
Kar M, Winther-Jensen B, Forsyth M, MacFarlane DR (2013) Chelating ionic liquids for reversible zinc electrochemistry. Phys Chem Chem Phys 15:7191–7197
Liu Z, Zein El Abedin S, Endres F (2013) Electrodeposition of zinc films from ionic liquids and ionic liquid/water mixtures. Electrochim Acta 89:635–643
Liu Z, Cui T, Pulletikurthi G, Lahiri A, Carstens T, Olschewski M, Endres F (2016) Dendrite-free nanocrystalline zinc electrodeposition from an ionic liquid containing nickel triflate for rechargeable Zn-based batteries. Angew Chem Int Ed 55:2889–2893
Periyapperuma K, Zhang Y, MacFarlane DR, Forsyth M, Pozo-Gonzalo C, Howlett PC (2017) Towards higher energy density redox-flow batteries: imidazolium ionic liquid for Zn electrochemistry in flow environment. ChemElectroChem 4:1051–1058
Dilasari B, Jung Y, Kwon K (2017) Effect of water on the stability of zinc in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquid. J Ind Eng Chem 45:375–379
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38:2520–2531
Chen S, Wu G, Sha M, Huang S (2007) Transition of ionic liquid [bmim][PF6] from liquid to high-melting-point crystal when confined in multiwalled carbon nanotubes. J Am Chem Soc 129:2416–2417
Chen S, Kobayashi K, Miyata Y, Imazu N, Saito T, Kitaura R, Shinohara H (2009) Morphology and melting behavior of ionic liquids inside single-walled carbon nanotubes. J Am Chem Soc 131:14850–14856
Im J, Cho SD, Kim MH, Jung YM, Kim HS, Park HS (2012) Anomalous thermal transition and crystallization of ionic liquids confined in graphene multilayers. Chem Commun 48:2015–2017
Wang Y-L, Laaksonen A, Lu Z-Y (2013) Influence of ionic liquid film thickness on ion pair distributions and orientations at graphene and vacuum interfaces. Phys Chem Chem Phys 15:13559–13569
Wang S, Li S, Cao Z, Yan T (2010) Molecular dynamic simulations of ionic liquids at graphite surface. J Phys Chem C 114:990–995
Wang Y-L, Laaksonen A (2014) Interfacial structure and orientation of confined ionic liquids on charged quartz surfaces. Phys Chem Chem Phys 16:23329–23339
Lin M-C, Gong M, Lu B, Wu Y, Wang D-Y, Guan M, Angell M, Chen C, Yang J, Hwang B-J, Dai H (2015) An ultrafast rechargeable aluminium-ion battery. Nature 520:324–328
Wang X, Fulvio PF, Baker GA, Veith GM, Unocic RR, Mahurin SM, Chi M, Dai S (2010) Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids. Chem Commun 46:4487–4489
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P, Taberna PL (2006) Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313:1760–1763
Largeot C, Portet C, Chmiola J, Taberna P-L, Gogotsi Y, Simon P (2008) Relation between the ion size and pore size for an electric double-layer capacitor. J Am Chem Soc 130:2730–2731
Elbourne A, McLean B, Voïtchovsky K, Warr GG, Atkin R (2016) Molecular resolution in situ imaging of spontaneous graphene exfoliation. J Phys Chem Letters 7:3118–3122
Liu Z, El Abedin SZ, Endres F (2015) Raman and FTIR spectroscopic studies of 1-ethyl-3-methylimidazolium trifluoromethylsulfonate, its mixtures with water and the solvation of zinc ions. ChemPhysChem 16:970–977
Hurisso BB, Lovelock KRJ, Licence P (2011) Amino acid-based ionic liquids: using XPS to probe the electronic environment via binding energies. Phys Chem Chem Phys 13:17737–17748
Blyth RIR, Buqa H, Netzer FP, Ramsey MG, Besenhard JO, Golob P, Winter M (2000) XPS studies of graphite electrode materials for lithium ion batteries. Appl Surf Sci 167:99–106
Briggs D, Beamson G (1993) XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal Chem 65:1517–1523
Briggs D, Beamson G (1992) Primary and secondary oxygen-induced C1s binding energy shifts in x-ray photoelectron spectroscopy of polymers. Anal Chem 64:1729–1736
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47–57
Shapouri Ghazvini M, Pulletikurthi G, Lahiri A, Endres F (2016) Electrochemical and spectroscopic studies of zinc acetate in 1-ethyl-3-methylimidazolium acetate for zinc electrodeposition. ChemElectroChem 3:598–604
Quilès F, Burneau A (1998) Infrared and Raman spectroscopic study of uranyl complexes: hydroxide and acetate derivatives in aqueous solution. Vib Spectrosc 18:61–75
Cabaço MI, Besnard M, Danten Y, Coutinho JAP (2011) Solubility of CO2 in 1-butyl-3-methyl-imidazolium-trifluoro acetate ionic liquid studied by Raman spectroscopy and DFT investigations. J Phys Chem B 115:3538–3550
Liu Z, Cui T, Lu T, Shapouri Ghazvini M, Endres F (2016) Anion effects on the solid/ionic liquid interface and the electrodeposition of zinc. J Phys Chem C 120:20224–20231
Simons TJ, Torriero AAJ, Howlett PC, MacFarlane DR, Forsyth M (2012) High current density, efficient cycling of Zn2+ in 1-ethyl-3-methylimidazolium dicyanamide ionic liquid: the effect of Zn2+ salt and water concentration. Electrochem Commun 18:119–122
Shao Q, Tang J, Lin Y, Li J, Qin F, Yuan J, Qin L-C (2015) Carbon nanotube spaced graphene aerogels with enhanced capacitance in aqueous and ionic liquid electrolytes. J Power Sources 278:751–759
Lewandowski A, Olejniczak A, Galinski M, Stepniak I (2010) Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J Power Sources 195:5814–5819
Fu C, Kuang Y, Huang Z, Wang X, Yin Y, Chen J, Zhou H (2011) Supercapacitor based on graphene and ionic liquid electrolyte. J Solid State Electrochem 15:2581–2585
Agiorgousis ML, Sun Y-Y, Zhang S (2017) The role of ionic liquid electrolyte in an aluminum–graphite electrochemical cell. ACS Energy Lett 2:689–693
Rothermel S, Meister P, Schmuelling G, Fromm O, Meyer H-W, Nowak S, Winter M, Placke T (2014) Dual-graphite cells based on the reversible intercalation of bis(trifluoromethanesulfonyl)imide anions from an ionic liquid electrolyte. Energy Environ Sci 7:3412–3423
Ujjain SK, Sahu V, Sharma RK, Singh G (2015) High performance, all solid state, flexible supercapacitor based on ionic liquid functionalized graphene. Electrochim Acta 157:245–251
Zhang F, Zhang T, Yang X, Zhang L, Leng K, Huang Y, Chen Y (2013) A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ Sci 6:1623–1632
Ye L, Liang Q, Lei Y, Yu X, Han C, Shen W, Huang Z-H, Kang F, Yang Q-H (2015) A high performance Li-ion capacitor constructed with Li4Ti5O12/C hybrid and porous graphene macroform. J Power Sources 282:174–178
Zheng JP (2009) High energy density electrochemical capacitors without consumption of electrolyte. J Electrochem Soc 156:A500–A505
Zheng JP (2003) The limitations of energy density of battery/double-layer capacitor asymmetric cells. J Electrochem Soc 150:A484–A492
Acknowledgements
The financial support by the BMBF project LUZI (BMBF: 03SF0499A) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Z., Li, G., Cui, T. et al. A battery-supercapacitor hybrid device composed of metallic zinc, a biodegradable ionic liquid electrolyte and graphite. J Solid State Electrochem 22, 91–101 (2018). https://doi.org/10.1007/s10008-017-3725-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3725-x