Abstract
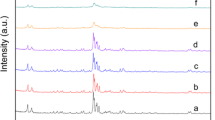
We report the synthesis and characterization of a new mesoporous cobalt oxide-infiltrated NaY zeolite prepared by ion-exchange route. The scanning electronic microscopy (SEM) image shows homogenous and uniform grains size distributions smaller than 1 μm, unlike to CoOx particles, elaborated under the same conditions. The energy dispersion spectroscopy (EDS) data confirm the presence of cobalt, oxygen, silicon, and aluminum. The X-ray diffraction indicates a partial crystallization of cobalt oxide and the formation of new phases. N2 adsorption-desorption measurement shows a high-specific surface area for the modified material (579 m2 g−1), with Barrett-Joyner-Halenda (BJH) pore diameters in the range (3–8 nm). The cyclic voltammetry indicates a typical faradic process, and the electrochemical impedance spectroscopy exhibits Warburg diffusion at low frequencies. The charge-discharge curve shows a clear improvement in the charge capacity of the modified material compared to CoOx, due to the increased specific surface area. The galvanostatic charge-discharge tests of the modified electrode exhibit a typical battery behavior preceded by a pseudo-capacitive phenomenon.








Similar content being viewed by others
References
Yang J, Liu H, Martens WN, Frost RL (2010) Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J Phys Chem C 114(1):111–119
Xiuyan X, Jinjun L, Zhengping H (2006) CeO2-Co3O4 catalysts for low-temperature CO oxidation. J Rare Earths 24:172–176
CoAC LJ, Serebrennikova I, Abel CM, Birss VI (2005) Structural and electrochemical studies of Co oxide films formed by the sol-gel technique. J Mat Sci 40(15):4039–4052
Wang L, Peng Z, Lei M, Fu X (2012) Solvothermal growth of cobalt oxide hexagon nanodiscs. Key Eng Mater 512–515:166–169.
Chen C, Cho M, Lee Y (2015) Electrochemical preparation and energy storage properties of nanoporous Co (OH)2 via pulse current deposition. J Mat Sci 50(19):6491–6497
Jena A, Munichandraiah N, Shivashankar SA (2012) Morphology controlled growth of meso-porous Co3O4 nanostructures and study of their electrochemical capacitive behavior. J Electrochem Soc 159(10):A1682–A1689
Feng J, Zeng HC (2003) Size-controlled growth of Co3O4 nanocubes. Chem Mater 15(14):2829–2835
Meher SK, Rao GR (2011) Effect of microwave on the nanowire morphology, optical, magnetic, and pseudo-capacitance behavior of Co3O4. J Phys Chem C 115:25543–25556
Nam KM, Shim JH, Han DW, et al. (2010) Syntheses and characterization of wurtzite CoO, rocksalt CoO, and spinel Co3O4 nanocrystals: their interconversion and tuning of phase and morphology. Chem Mater 22(15):4446–4454
Cui L, Li J, Zhang XG (2009) Preparation and properties of Co3O4 nanorods as supercapacitors material. J Appl Electrochem 39:1871–1876
Natile MM, Glisenti A (2003) New NiO/Co3O4 and Fe2O3/Co3O4 nanocomposite catalysts: synthesis and characterization. Chem Mater 15(13):2502–2510
Musat V, Fortunato E, Botelho do Rego AM, Monteiro R (2008) Sol–gel cobalt oxide–silica nanocomposite thin films for gas sensing applications. Thin Solid Films 516:1499–1502
Rosen J, Hutchings GS, Jiao F (2013) Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst. J Am Chem Soc 135(11):4516–4521
Vezvaie M, Kalisvaart P, Fritzsche H, et al. (2014) The penetration depth of chemical reactions in a thin-film Co3O4 supercapacitor electrode. J Electrochem Soc 161(5):A798–A802
Yao W, Yang J, Wang J, Nuli Y (2008) Multilayered cobalt oxide platelets for negative electrode material of a lithium-ion battery. J Electrochem Soc 155(12):A903–A908
Xie Y, Dong F, Heinbuch S, Roccab JJ, Bernstein ER (2010) Oxidation reactions on neutral cobalt oxide clusters: experimental and theoretical studies. Phys Chem Chem Phys 12:947–959
Liao L, Zhang Q, Su Z, et al. (2014) Efficient solar water-splitting using a nanocrystalline CoO photocatalyst. Nat Nanotechnol 9:1–5
Patnaik P (2003) Handbook of inorganic chemicals. McGraw-Hill, New York
Schweitzer GK, Pesterfield LL (2010) The aqueous chemistry of the elements. Oxford University Press, New York
Zhuiykov S (2007) Semiconductor sensor using cobalt oxyhydroxide CoOOH for carbon monoxide detection at low temperatures. Mater Forum 31:144–151
Han Y, Dong L, Feng J, Li D, Li X, Liu S (2015) Cobalt oxide modified porous carbon anode enhancing electrochemical performance for Li-ion batteries. Electrochim Acta 167:246–253
Khan IA, Nasim F, Choucair M, Ullah S, Badshah A, Nadeem MA (2016) Cobalt oxide nanoparticle embedded N-CNTs: lithium ion battery applications. RSC Adv 6:1129–1135
Corma A (2003) State of the art and future challenges of zeolites as catalysts. J Catal 216:298–312
Xu X, Wang J, Long Y (2006) Zeolite-based materials for gas sensors. Sensors 6:1751–1764
Lutz W (2014) Zeolite Y: synthesis, modification, and properties—a case revisited. Adv Mater SciEng 2014:1–20
Chester AW, Derouane EG (2009) Zeolite characterization and catalysis: a tutorial. Springer, Heidelberg
Thoret J, Man PP, Fraissard J (1995) Insertion of vanadium or molybdenum as oxides in LaNaY zeolite: comparison with nay. J Chem Soc Faraday Trans 91(6):1037–1043
Chen H, Matsumoto A, Nishimiya N, Tsutsumi K (1999) Preparation and characterization of TiO2 incorporated Y-zeolite. Colloid Surf A 157:295–305
Nicholson RS (1965) Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal Chem 37(11):1351–1354
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications, 2nd edn. John Wiley& Sons, New York
Abdi A, Trari M (2013) Investigation on photoelectrochemical and pseudo-capacitance properties of the non-stoichiometric hematite α-Fe2O3 elaborated by sol–gel. Electrochim Acta 111:869–875
Cerny J, Micka K (1989) Voltametric study of an iron electrode in alkaline electrolytes. J Power Sources 25:111–122
Hang BT, Yoon SH, Okada S, Yamaki JI (2007) Effect of metal-sulfide additives on electrochemical properties of nano-sized Fe2O3-loaded carbon for Fe/air battery anodes. J Power Sources 168(2):522–532
Raistrick ID, Franceschetti DR, Macdonald JR (2003) In. Barsoukov E, Macdonald JR ed) Impedance spectroscopy, 2nd edn. John Wiley & Sons, New Jersey
Keswani M, Raghavan S, Deymier P (2013) A novel way of detecting transient cavitation near a solid surface during megasonic cleaning using electrochemical impedance spectroscopy. Microelectron Eng 108:11–15
Lasia A (2014) Electrochemical impedance spectroscopy and its applications. Springer-Verlag, New York
Feliu Jr S, Barajas R, Bastidas JM, Morcillo M, Feliu S (1993) In. Scully JR, Silverman DC, Kendig MW (eds) Electrochemical impedance: analysis and interpretation, ASTM STP 1188. American Society for Testing and Materials, Philadelphia
Lide DR (ed) (2004) CRC handbook of chemistry and physics, 84th edn. Florida, CRC Press, Boca Raton
Flury M, Gimmi T (2002) In: Dane JH, Topp GC (eds) Methods of soil analysis, part 4-physical methods, Soil Science Society of America, Madison, WI
Zhang SM, Zhang JX, SJ X, Yuan XJ, He BC (2015) Li ion diffusivity and electrochemical properties of FePO4 nanoparticles acted directly as cathode materials in lithium ion rechargeable batteries. Electrochim Acta 88:287–293
Green M, Fielder E, Scrosati B, Wachtler M, Moreno JS (2003) Structured silicon anodes for lithium battery applications. Electrochem Solid-State Lett 6(5):A75–A79
Jorcin JB, Orazemb ME, Pébère N, Tribollet B (2006) CPE analysis by local electrochemical impedance spectroscopy. Electrochim Acta 51:1473–1479
Acknowledgments
The authors thank Dr. J. Douglade, Dr. A. May, Dr. S. Belkhiri, and Dr. B. Bellal, for providing XRD analysis, SEM images, BET analysis, and UV-visible NIR spectra, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdi, A., Aaboubi, O. & Trari, M. Investigation on structural, morphological, and electrochemical properties of mesoporous cobalt oxide-infiltrated NaY zeolite. J Solid State Electrochem 21, 383–390 (2017). https://doi.org/10.1007/s10008-016-3378-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3378-1